Substituting Cis-1, 2-dicyano vinyl and synthesis process thereof
A technology for the synthesis of ethylene dicyanide, which is applied to aryl-substituted cis-1, can solve the problems of harm to operators and the environment, low total yield, and difficult separation, and achieve low toxicity of raw materials, high utilization rate, and wide application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
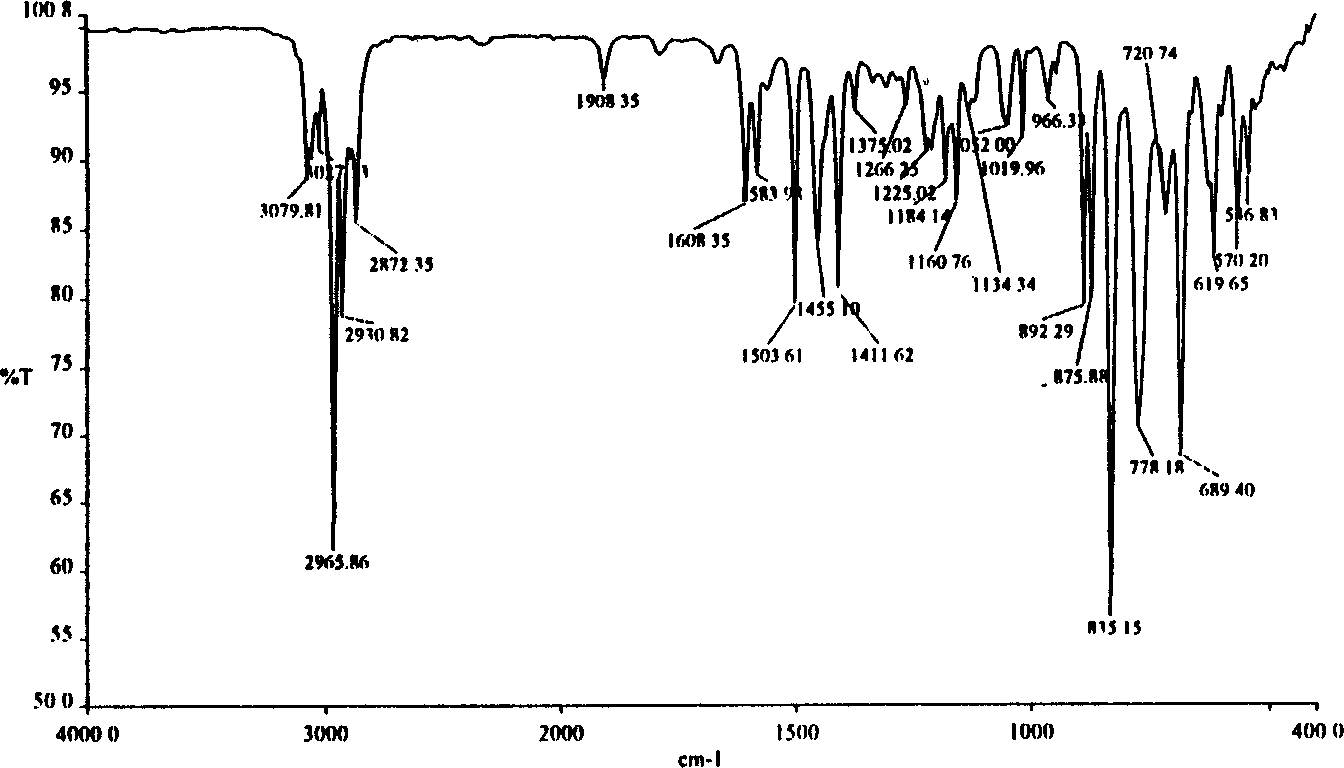
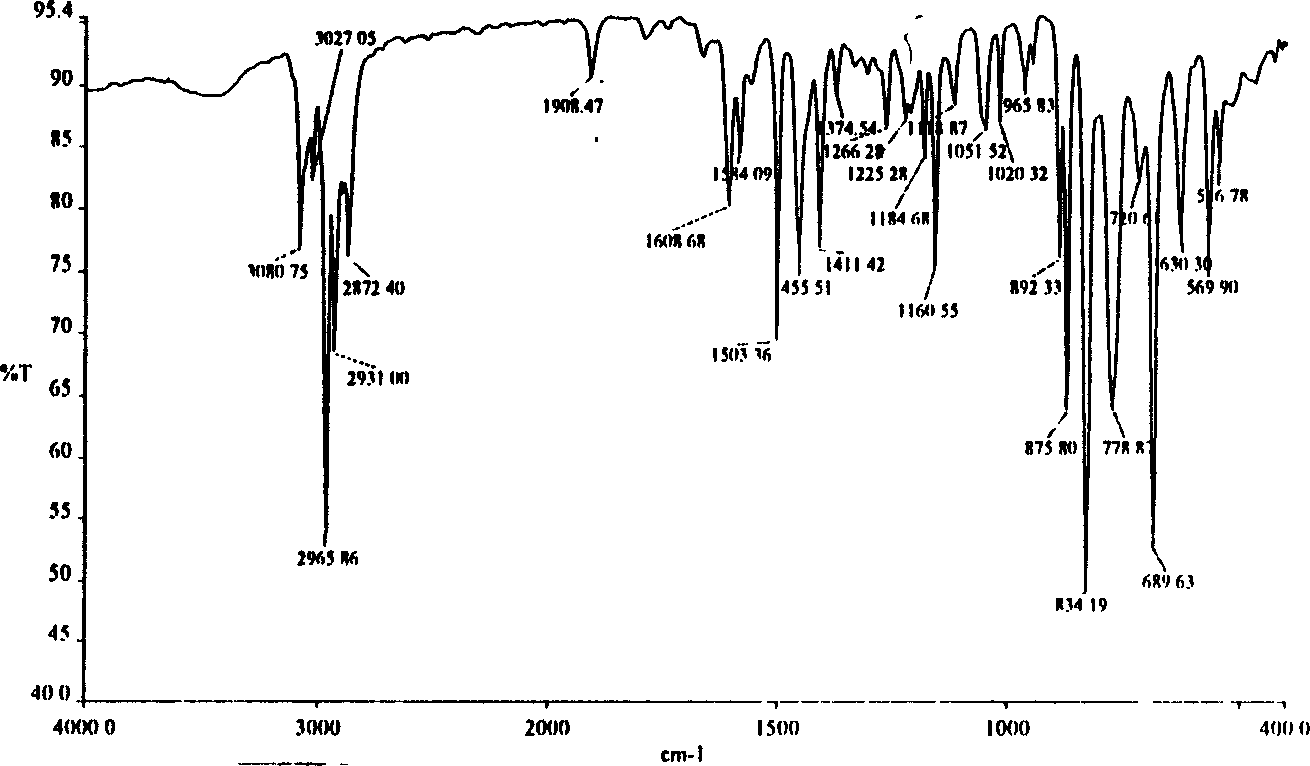
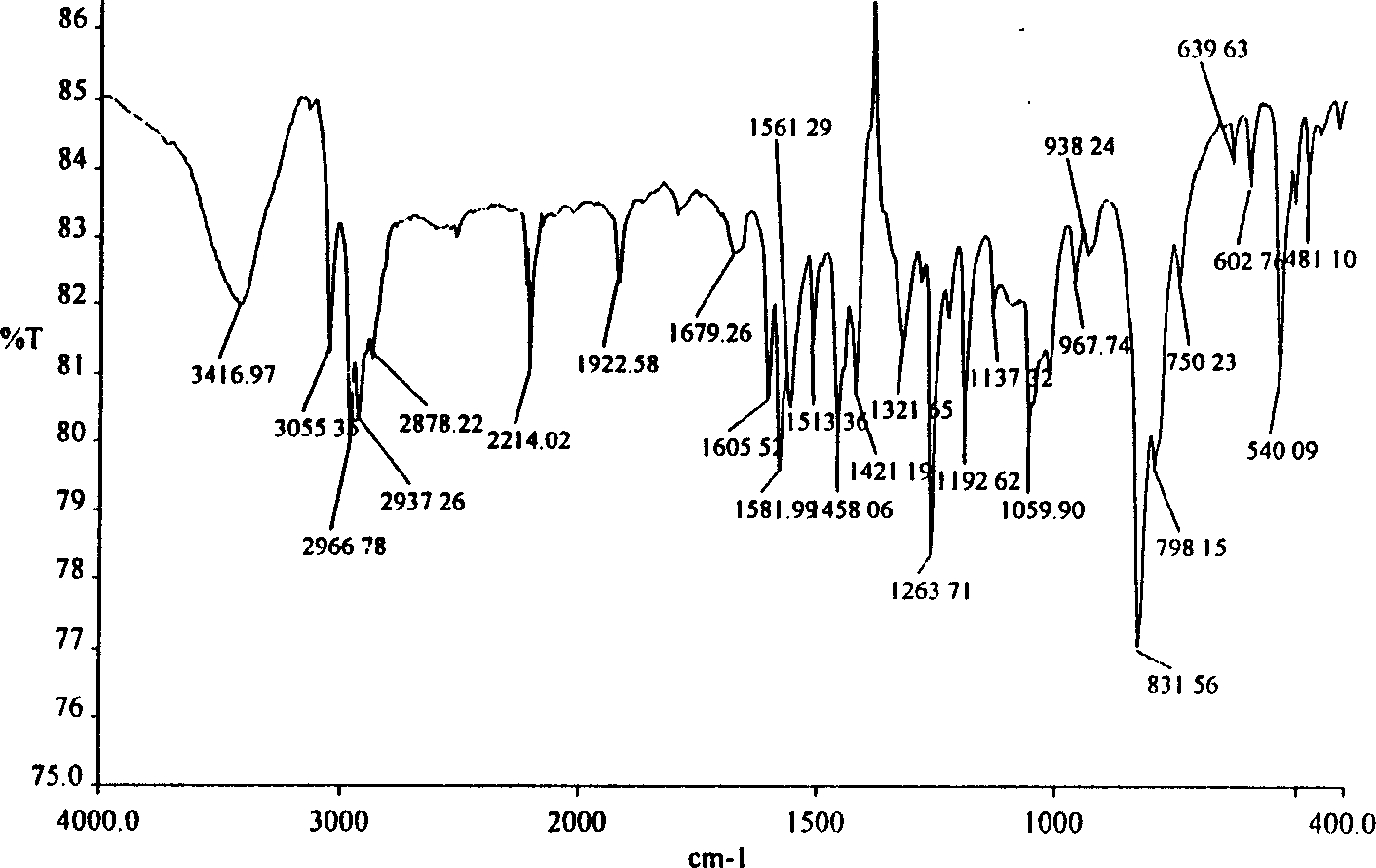
Image
Examples
Embodiment 1
[0029] (1) Synthesis of trans-1,2-dibromo-(4-ethylbenzene)ethylene (1a) and cis-1,2-dibromo-(4-ethylbenzene)ethylene (1a')
[0030] Add 200ml of dry CCl to the reaction flask 4 , 25.0ml of 4-ethylphenylacetylene, 10ml of bromine and 100ml of dry CCl with a dropping funnel 4 The mixed solution of the mixture was slowly dropped into the above-mentioned solution. After the drop was completed, the stirring reaction was continued at room temperature for 4 hours. The reaction solution was washed and the solvent was evaporated to obtain the product trans-1,2-dibromo-(4-ethylbenzene)ethylene ( 1a) and cis-1,2-dibromo-(4-ethylbenzene)ethylene (1a') mixture, the mixture of the two is directly used in the next step reaction, and can also be further purified by column chromatography, weighed, and calculate the yield , Determination of the gas mass spectrometry and infrared spectrum of the product (as in Table 1).
[0031] Table 1 1,2-dibromo-(4-ethylbenzene)ethylene (trans 1a and cis 1a')...
Embodiment 2
[0072] (1) Synthesis of trans-1,2-dibromo-(4-butylbenzene)ethylene (1b) and cis-1,2-dibromo-(4-butylbenzene)ethylene (1b')
[0073] The 4-ethylphenylacetylene in the first step of Example 1 is replaced by equimolar 4-butylphenylacetylene, and the reaction under the same conditions produces trans-1,2-dibromo-(4-butylbenzene) Ethylene (1b) and cis-1,2-dibromo-(4-butylphenyl)ethylene (1b'), the mixture of the two was directly used in the next reaction. Column chromatography can also be used for further purification, weighing, calculating the yield, and measuring the gas-frequency spectrum and infrared spectrum of the product (as shown in Table 1).
[0074] (2) Synthesis of trans-1,2-dicyano-(4-butylbenzene)ethylene (2b) and cis-1,2-dicyano-(4-butylbenzene)ethylene (2b')
[0075] Replace the mixture of trans-1,2-dibromo-(4-ethylbenzene)ethylene and cis-1,2-dibromo-(4-ethylbenzene)ethylene in the second step of Example 1 with trans-1 , 2-dibromo-(4-butylphenyl)ethylene and cis-1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com