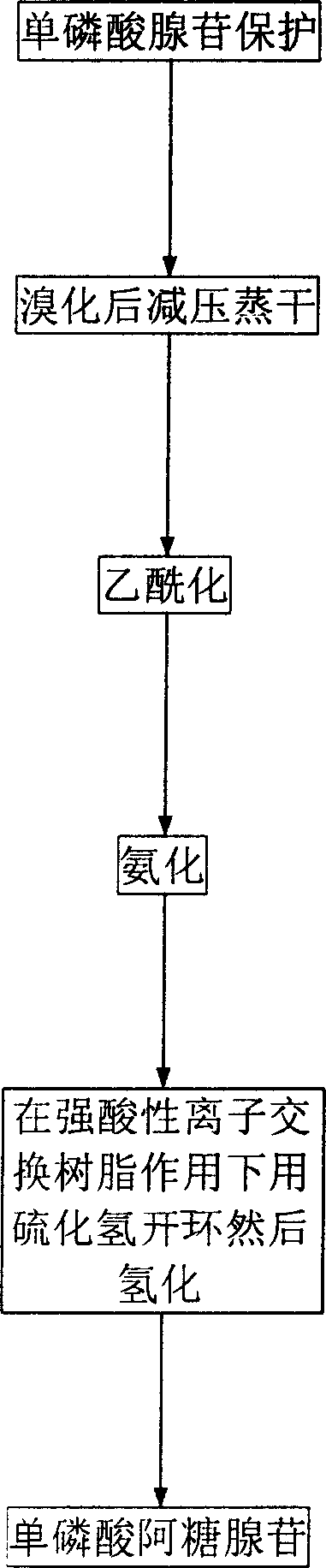
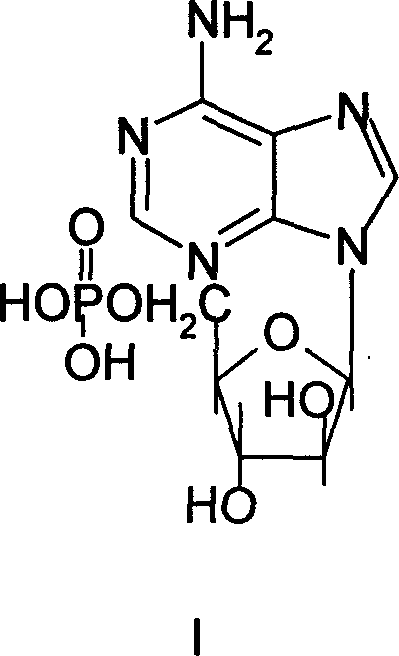
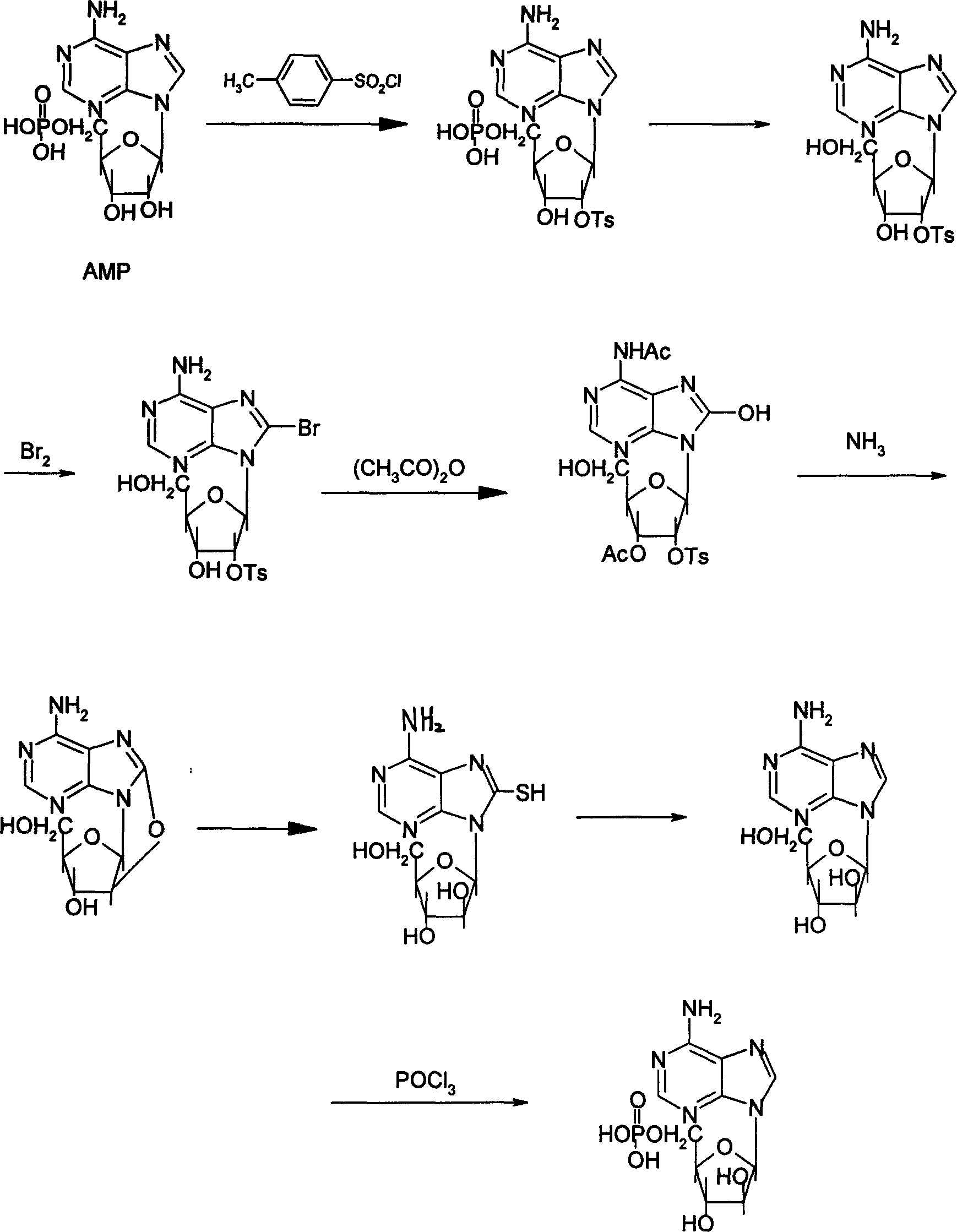
Synthesis process of adenosine aose monophosphate
A technology of adenosine vidarabine monophosphate and adenosine monophosphate, which is applied in the field of synthesis technology for the preparation of antiviral drug adenosine monophosphate, can solve problems such as unfavorable industrial production, achieve simplified operation, increase yield, and operate simplified effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] (1) Preparation of 2'-oxygen-p-toluenesulfonyl-5'-phosphate adenosine (II)
[0023] Add 34.7 grams of 5'-AMP to a mixed solution of 150 milliliters of dioxane and 350 milliliters of 1N sodium hydroxide; After stirring and reacting for 15 hours, 35 ml of 6N hydrochloric acid was added to adjust the pH to 4.0. The precipitated crystals were collected by filtration to obtain 46.4 g of crystalline powder II.
[0024] (2) Preparation of 8-bromo-2'-oxygen-p-toluenesulfonyl ester-5'-phosphate adenosine (III)
[0025] In 240 ml of 2 M sodium acetate (pH4) solution, add 46 g of II and cool to 0-5°C. Then 19 ml of bromine was added dropwise to this solution, maintaining 0-5°C. Stir at this temperature for 18 hours. At 0-5°C, 28 g of sodium bisulfite was added to the reaction solution. After stirring for 15 minutes, the pH was adjusted to 4.0 with 5 N NaOH (about 90-100 mL). Evaporated to dryness under reduced pressure, the obtained solid was directly used in the next reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com