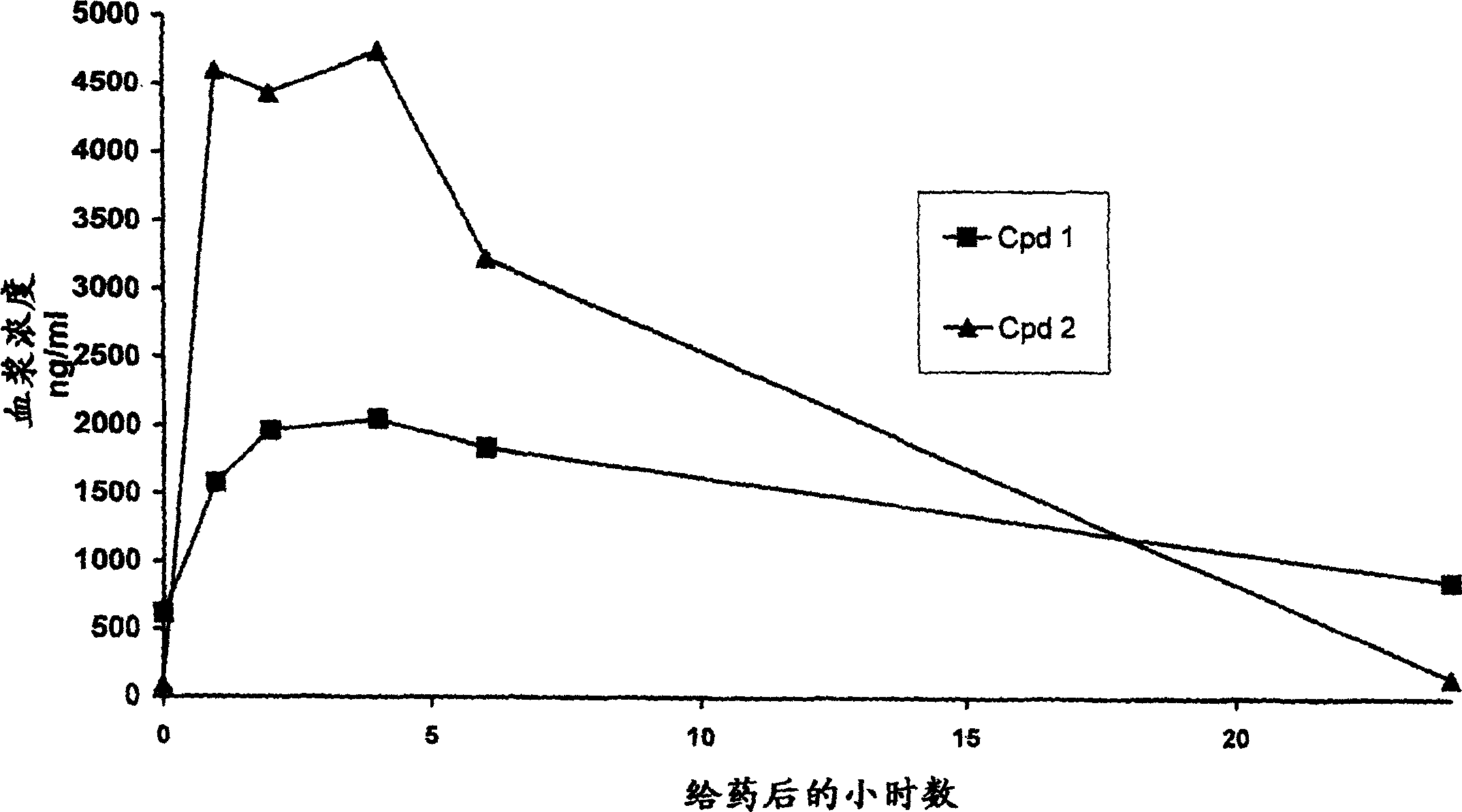
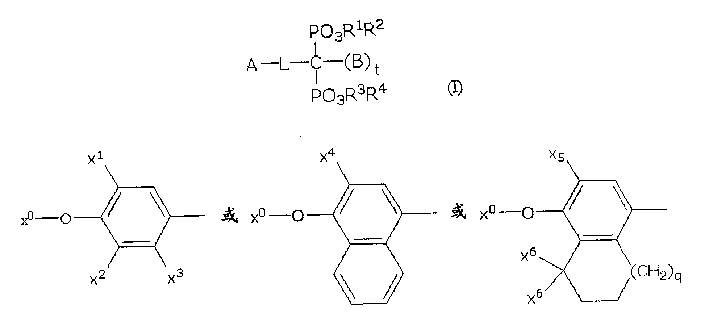
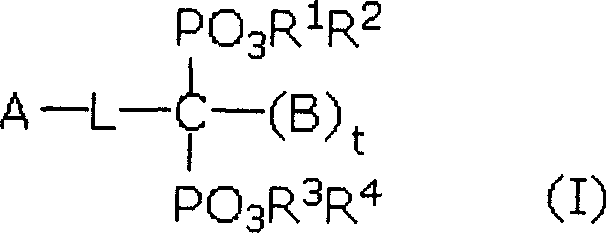Pharmaceutical compounds
A compound and drug technology, applied in the field of drug compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
[0148] Examples 1-4 of the present application describe the synthesis of the following compounds:
[0149]
[0150] in:
[0151] -X 0 is H, an alkyl group having 1 to 4 carbon atoms;
[0152] -X 4 is optionally substituted benzyl (Bn).
[0153] Examples 5-9 describe the osteoanabolic activity of compounds of formula (I).
Embodiment 1
[0154] Example 1: Tetraethyl 2-(3-benzyl-4-hydroxynaphthyl)vinylidene-1,1-diphosphonate
[0155]
[0156] a) 3-benzyl-4-hydroxynaphthaldehyde
[0157] Add potassium tert-butoxide (36.0 g, 0.32 mol) to a solution of 1-tetralone (23.4 g, 0.16 mol) and benzaldehyde (16.96 g, 0.16 mol) in tert-butanol (1600 ml), and make the resulting mixture under nitrogen Reflux overnight. The mixture was cooled, acidified with dilute HCl solution, and concentrated in vacuo to remove tert-butanol. The concentrated aqueous phase was extracted with ethyl acetate, the organic phase was evaporated and the residue obtained was purified by column chromatography to obtain 34 g of 2-benzyl-1-naphthol.
[0158] Add anhydrous tin chloride (21.38g, 82.1mmol) to a solution of 2-benzyl-1-naphthol (10.67g, 45.6mmol) in 60ml of dichloromethane at 0°C, and then add 1,1 dichloromethyl Methyl ether (7.92 g, 68.86 mmol). The resulting mixture was stirred at 0 °C for 30 minutes, then poured into ice water. ...
Embodiment 2
[0169] Example 2: Tetraisopropyl 2-(3-benzyl-4-hydroxynaphthyl)vinylidene-1,1-diphosphonate
[0170]
[0171] Titanium tetrachloride (10.89 g, 57.3 mmol) was added dropwise to 60 ml of anhydrous tetrahydrofuran while stirring. The following compounds were then added successively at 5 minute intervals while stirring at 0-5°C: 3-benzyl-4-hydroxynaphthaldehyde (5.0 g, 19.1 mmol), followed by tetraisopropylmethylene diphosphate ( 7.9 g, 22.9 mmol), and finally N-methylmorpholine (11.58 g, 114.6 mmol). After the addition was complete, the reaction was stirred at room temperature for 3 hours, then ice water was added. The quenched reaction mixture was extracted with diethyl ether and the ether extract was back extracted with brine to neutral pH and concentrated in vacuo to give a residue which was triturated in a mixture of petroleum ether and diethyl ether and then dissolved in tert-butyl methyl ether Purification by recrystallization afforded 4.1 g (37%) of the title compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com