Gene transfer into primate embryonic stem cells using VSV-G pseudo type simian immunodeficiency virus vectors
A technology of immunodeficiency virus and embryonic stem cells, applied to embryonic cells, using vectors to introduce foreign genetic material, viruses/bacteriophages, etc., can solve problems such as difficulties in gene introduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
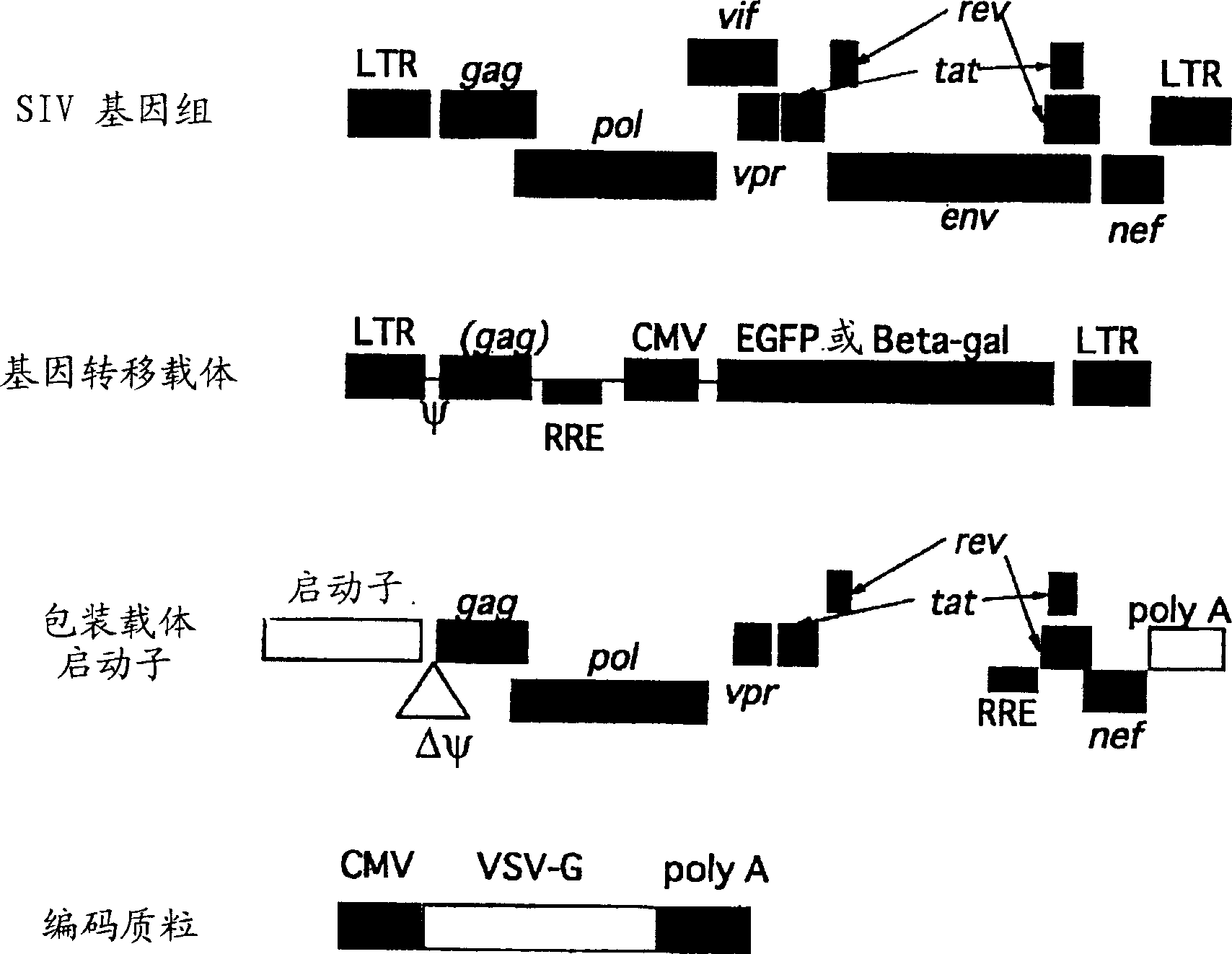
[0100] [Example 1] Construction of SIV vector
[0101] The non-pathogenic African green monkey (African green monkey) immunodeficiency virus clone - SIVagmTYO1 was used to construct the vector system. figure 1 Represents an outline of the vector system. The sequence numbers of all nucleotides are represented by the transcription start point of viral RNA+1 below. As a plasmid, pSA212 inserted with SIVagmTYO1 was used (J. Viol., vol. 64, pp307-312, 1990). And all ligation reactions were carried out according to the instructions attached to Ligation High (Toyobo).
[0102] a. Construction of packaging vector
[0103]First, use primers 1F (SEQ ID NO: 1) and 1R (SEQ ID NO: 2) and use pSA212 as a template to obtain the region (5337-5770) corresponding to the first exon of tat / rev and vif by PCR of DNA fragments. An EcoRI restriction enzyme site was added to the PCR primer, thereby preparing a DNA fragment with an EcoRI site at the 3' end. After digestion with BglII and EcoR...
Embodiment 2
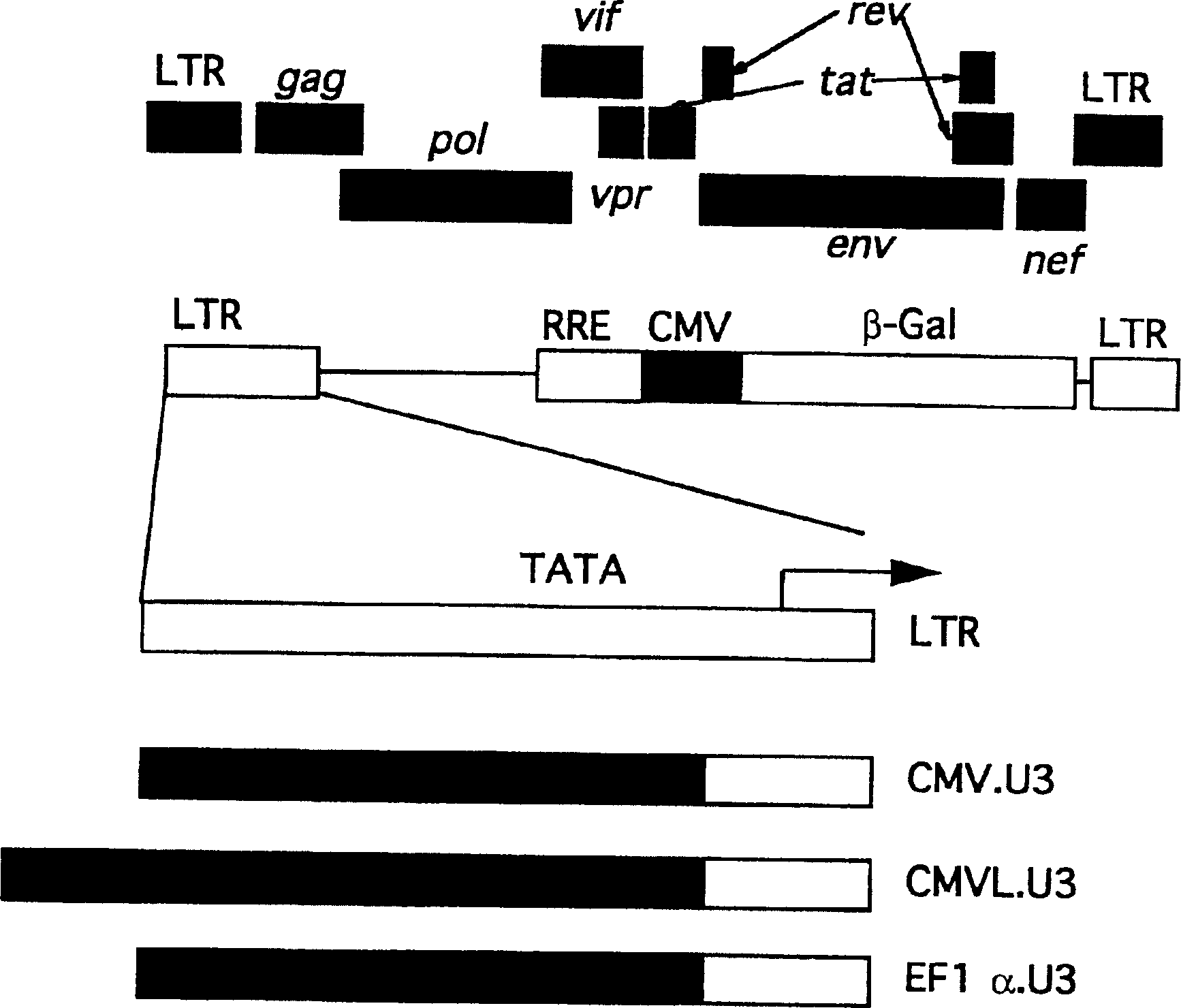
[0107] [Example 2] Modification of 5'LTR
[0108] The 5'LTR transcriptional activity of lentiviruses generally depends on the presence of Tat protein, a virus-derived factor. Therefore, in order to eliminate the dependence on Tat and increase the titer of the vector by substituting it with a highly transcriptionally active promoter sequence, a SIVagm gene transfer vector was prepared in which the 5'LTR promoter sequence--U3 region was replaced by other Promoter sequence replacement ( figure 2 ).
[0109] The 5'LTR was replaced with a chimeric promoter by PCR amplification using primers 9-1F-3F (SEQ ID NO: 21-23) and primer 9R (SEQ ID NO: 24) using pSA212 as a template Fragment comprising the region downstream of the TATA box of the 5'LTR up to the gag region (9039-9170+1-982). Then, a fragment containing the CMVL promoter (from pCI (Promega), 1-721) was amplified by PCR using primers 10-1F (SEQ ID NO: 25) and 10-1R (SEQ ID NO: 26) using pCI as a template ; A fragment comp...
Embodiment 3
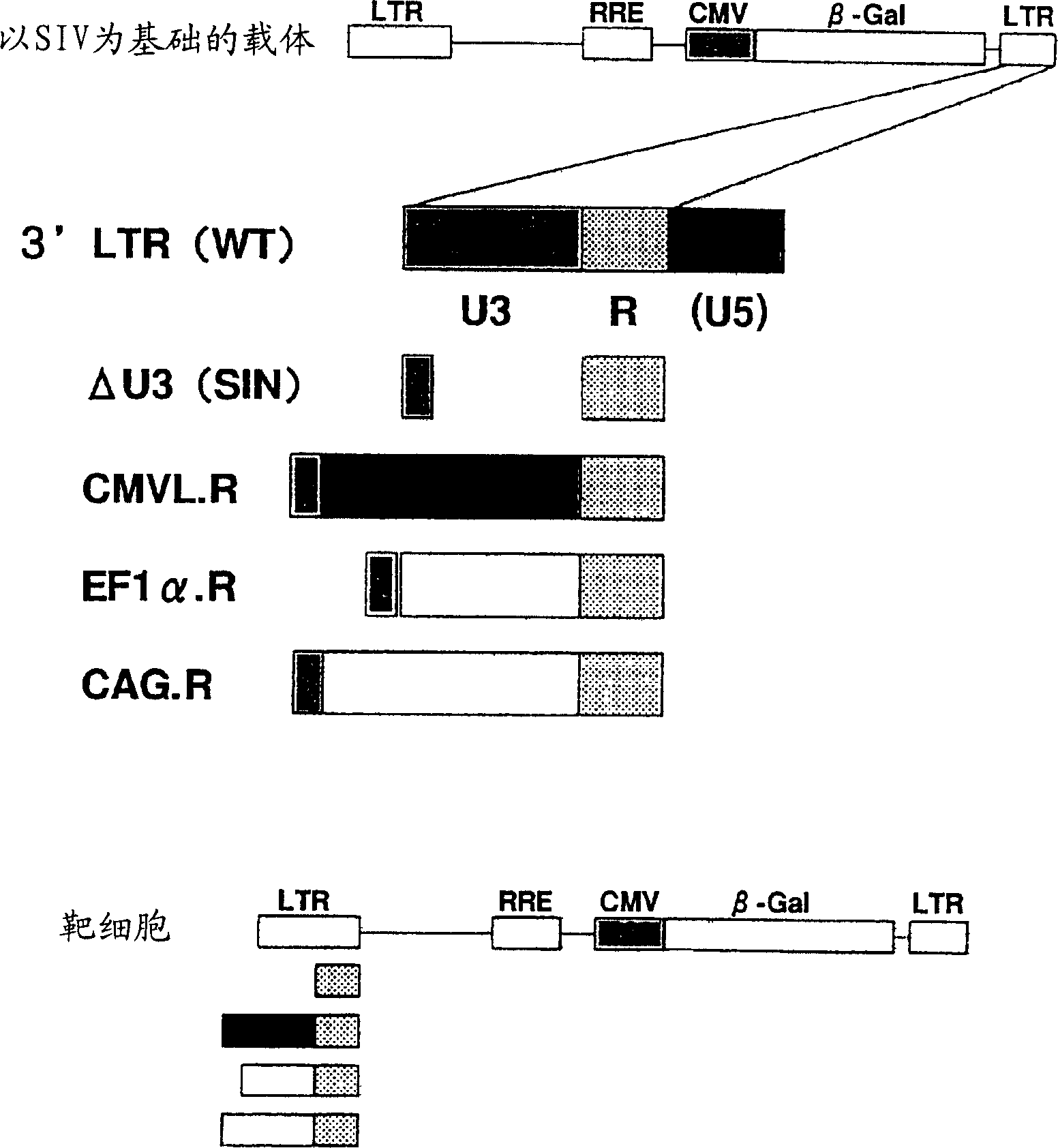
[0110] [Example 3] Modification of 3'LTR
[0111] A partial sequence of the 3'LTR is excised to prevent full-length vector mRNA from being transcribed in target cells, thereby constructing a self-inactivating (SIV) vector with improved safety. In the lentiviral vector, it has been proved that the promoter sequence contained in the 3'LTR region - the U3 region can be integrated into the U3 promoter region of the 5'LTR during reverse transcription in the target cell. Therefore, in the genome of the target cell, the gene The U3 region contained in the 3'LTR region of the transfer vector plasmid can be used as the 5'LTR U3 promoter region associated with gene expression ( image 3 ). Therefore, a vector in which the 3'LTR U3 region of the SIVagm gene transfer vector was substituted with other promoter sequences was prepared ( image 3 ). In addition, in order to study whether the 5'LTR in the target cells can delete the promoter sequence, a vector that deletes the 3'LTR U3 regi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com