Specific antibody of tumor-associated antigen SM5-1 and use thereof
A specific and antibody technology, applied in the field of tumor immunology, which can solve the problems of unsatisfactory specificity and neutralization ability of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
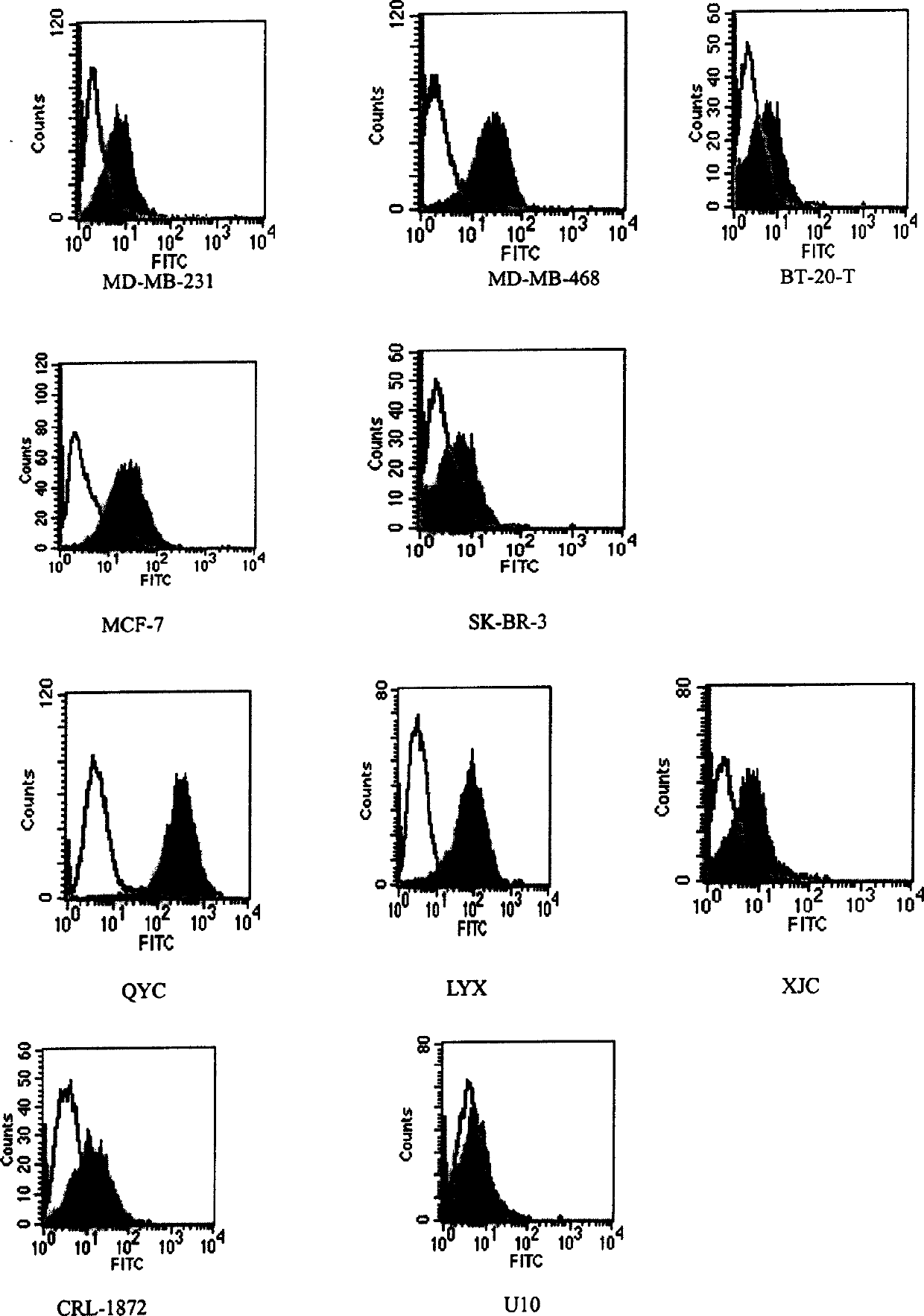
[0147] Screening and identification of huSM5-1 antigen
[0148] 1. Construction of cDNA library of hepatocellular carcinoma cell line QYC
[0149] Total RNA was extracted from human hepatocellular carcinoma cells QYC (purchased from Shanghai International Cooperative Cancer Institute) with Trizol reagent. Isolate mRNA and synthesize cDNA according to the method reported in the literature (Marken JS. PNAS, 1992, 89: 3503-3507), fill in and connect with the adapter of the BstXI restriction site, and clone into a mammalian transient expression vector after digestion with BstXI Escherichia coli MC1061 / P3 (purchased from Invitrogen) was electrotransformed into pCDM8 (purchased from Invitrogen) to form a cDNA library.
[0150] 2. Library expression and screening
[0151] The cDNA library constructed above was used to transfect COS-7 cells (Invitrogen) according to lipofectin method. After 12 hours, trypsinize the cells and place them on a new culture dish. 72 hours ...
Embodiment 2
[0155] Screening of huSM5-1 variable region gene from human antibody library
[0156] According to Marks et al. J. Mol. Biol., 222, 581-597; Hoogenboom and Winte, J. Mol. Biol., 227, 381-388; Haidaris CG et al., J Immunol Methods. 2001 Nov 1; 257(1-2 ): 185-202; Griffiths, A.D. et al. EMBO J., 13, 3245-3260 (1994); Nissim, A. et al. EMBO J., 13, 692-698 (1994) to construct human antibody libraries.
[0157] Add 1 ml of the revived antibody library strain to 14 ml of fresh LB medium, and culture in a 50 ml Erlenmeyer flask at 37°C for 16 hours.
[0158] The bacterial solution was centrifuged at 12,000 rpm for 10 minutes at high speed, and the supernatant was transferred to a sterile 50 ml centrifuge tube, and stored for later use. Its titer should be 2×10 11 above. A 25 ml cell culture flask was coated with the antigen A230 (SM5-1) purified in Example 1. Add no less than 3×10 10 Phage particles were incubated at 37°C for 1 hour. Then, the liquid in the bottl...
Embodiment 3
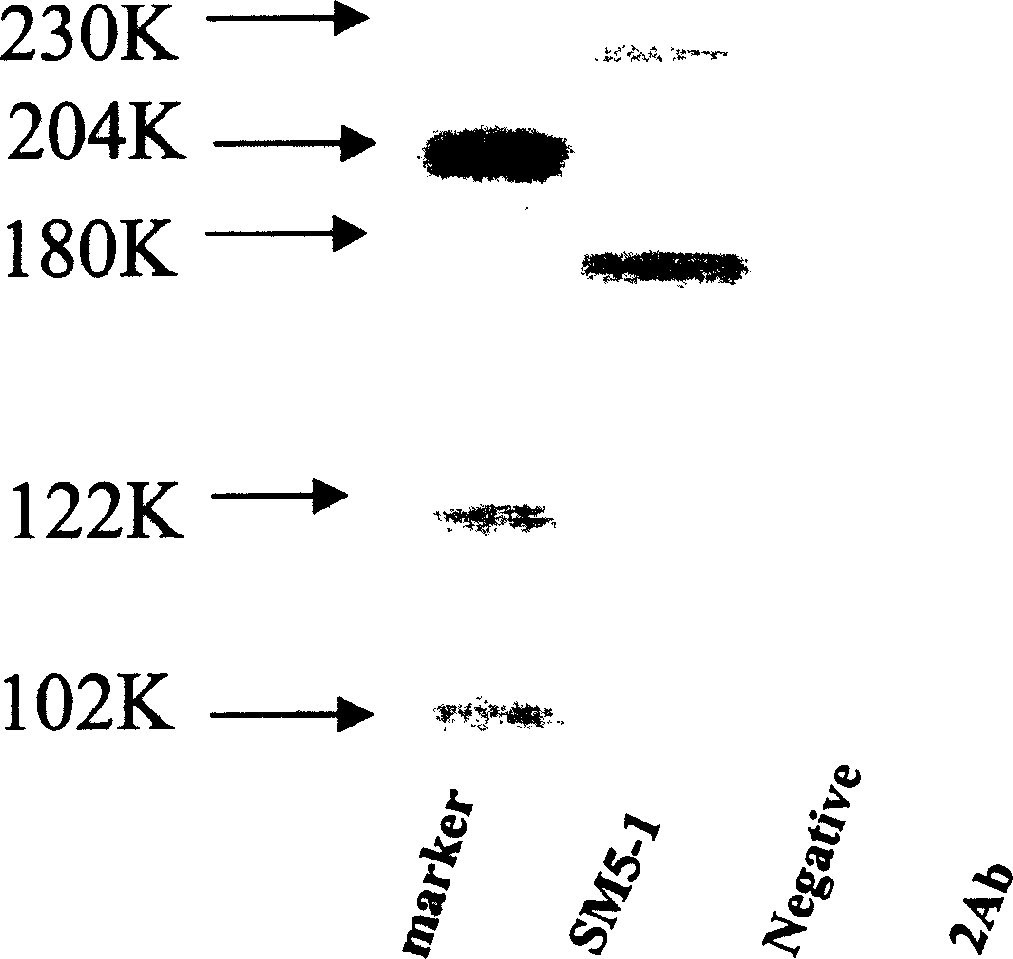
[0166] Preparation of huSM5-1 monoclonal antibody
[0167] 1. Construction of huSM5-1 expression vector
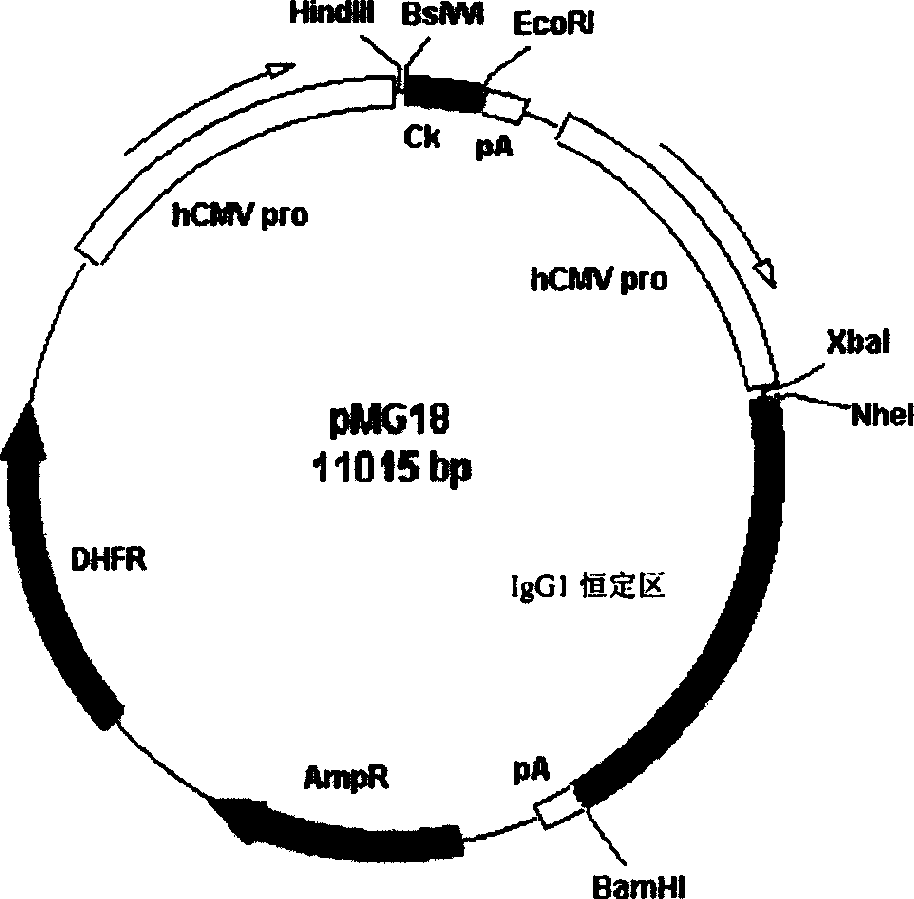
[0168] The XbaI restriction site and the signal peptide of monoclonal antibody OKT3 were added to the 5' end of the heavy chain variable region gene of huSM5-1 by PCR, and the NheI restriction site was added to the 3' end. The amino acid sequence of the signal peptide of monoclonal antibody OKT3 is MDFQVQIFSFLLISASVIISRG (SEQ ID NO: 13), and its nucleotide sequence is ATGGATTTTCAGGTGCAGATTTTCAGCTTCCTGCTAATCAGTGCCTCAGTCATAATATCCAGAGGAG (SEQ ID NO: 14). The above PCR products were cloned into pGEM-T vector for sequencing confirmation. The heavy chain variable region was digested with XbaI and NheI and inserted into the pMG18-3K expression vector shown in Figure 1 (from Development of tools for environmental monitoring based on incp-9 plasma sequences. A. Greated, R. Krasowiak, M. Titok, C.M.Thomas school of biological sciences, university of Bermingham, Edgba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com