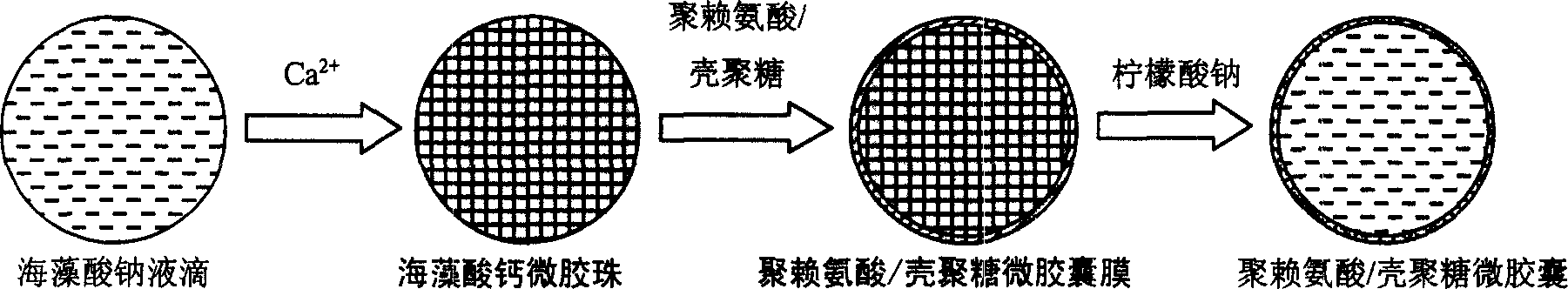
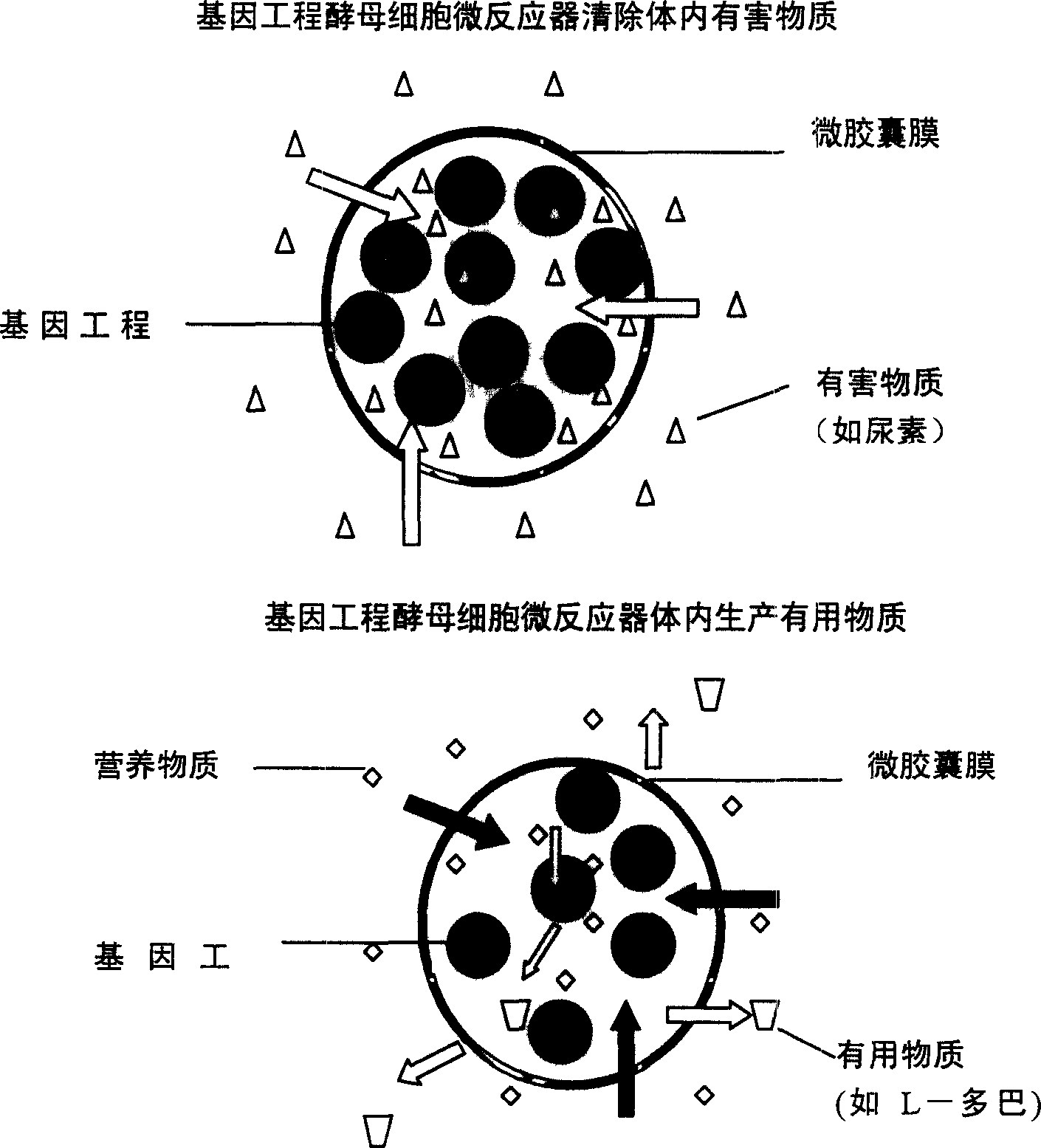
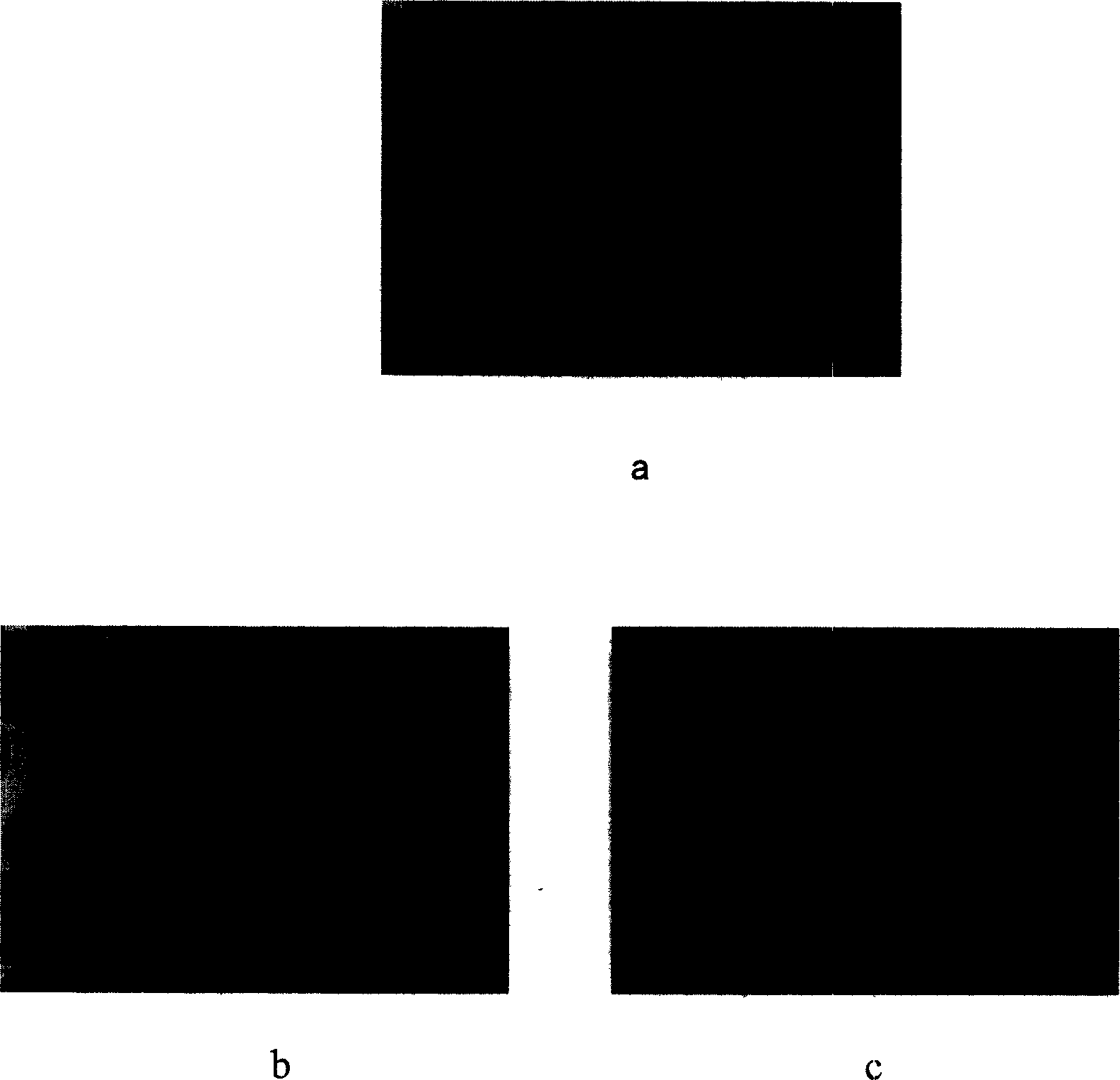Microencapsulated saccharomyces and its application
A technology of yeast and microencapsulation, applied in the direction of microcapsules, fungi, digestive system, etc., can solve the problems of no biological activity, no secretion, and acceptance by patients with odor, and achieve the effect of improving cell survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Example 1: APCA microcapsules containing yeast were prepared under physiological conditions. During the preparation process, the survival rate of yeast was 100%, and the yeast content was 10% 8 One / ml microcapsules, the particle size of the microcapsules is precisely controllable in the range of 100-1000μm.
example 2
[0023] Example 2: After the APCA microcapsules containing yeast were treated with simulated gastric and intestinal fluids, the microcapsules remained intact without damage (see image 3 ).
example 3
[0024] Example 3: After the APCA microcapsules containing yeast are treated with simulated gastric and intestinal fluid, the physiological breaking solution dissolves the microcapsules and releases the yeast. The number of surviving yeast is measured by the dilution plate method, and the yeast count is compared with that before untreated Compared with free yeast, the survival rate of cells in the capsule is increased by more than 200 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com