Non-aqueous electrolyte battery
A non-aqueous electrolyte and battery technology, applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolyte batteries, non-aqueous electrolytes, etc., can solve the problems of insufficient improvement of cycle characteristics, reduced battery characteristics, and weak covering films
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
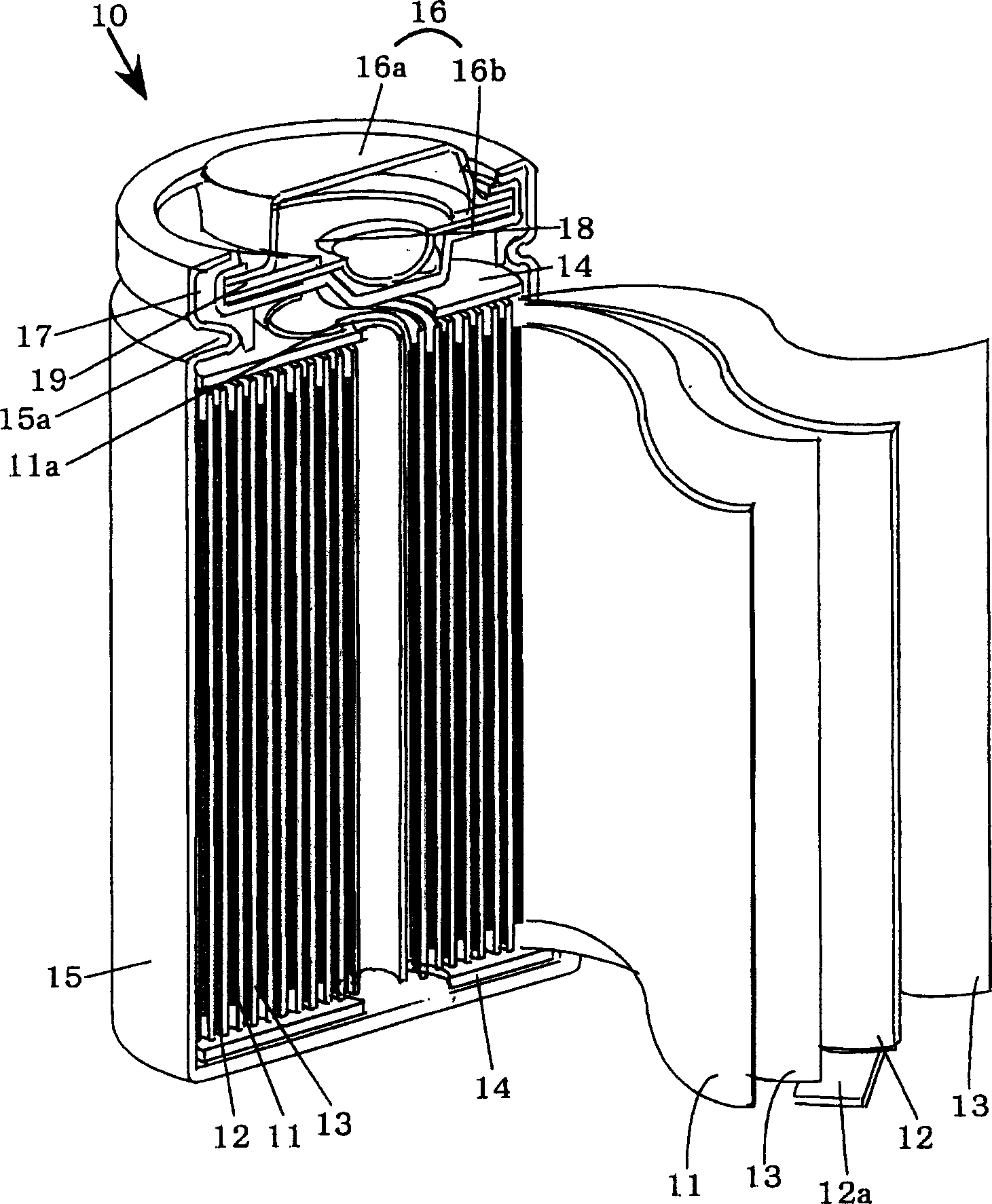
[0022] Hereinafter, an embodiment of the present invention will be described, but the present invention is not limited to the embodiment, and can be implemented with appropriate changes within a range that does not change the object of the present invention. and, figure 1 It is a partially cutaway perspective view schematically showing a state in which the main part of the non-aqueous electrolyte battery of the present invention is cut longitudinally.
[0023] 1. Fabrication of positive electrode
[0024] Lithium cobalt oxide (LiCoO 2 ) powder, acetylene black as a conductive agent, and fluororesin as a binder were mixed at a mass ratio of 90:5:5 to prepare a positive electrode mixture. N-methyl-2-pyrrolidone (NMP) was added to this positive electrode mixture and mixed to form a slurry. Thereafter, the slurry was applied to both surfaces of a positive electrode current collector made of aluminum foil by a doctor blade method to form a positive electrode mixture layer. Then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com