Oleanolic acid couple derivatives and their pharmaceutical use
A compound and a representative technology used in the preparation of drugs that inhibit apoptosis or selectively induce apoptosis of liver tumor cells can solve the problems of low bioavailability and unsatisfactory curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
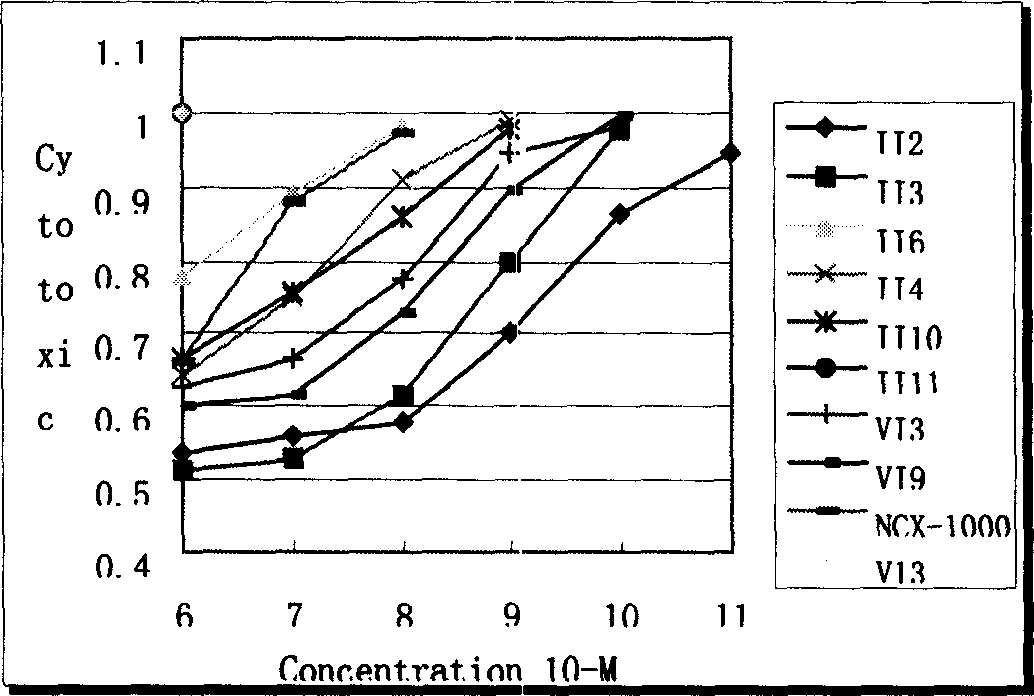
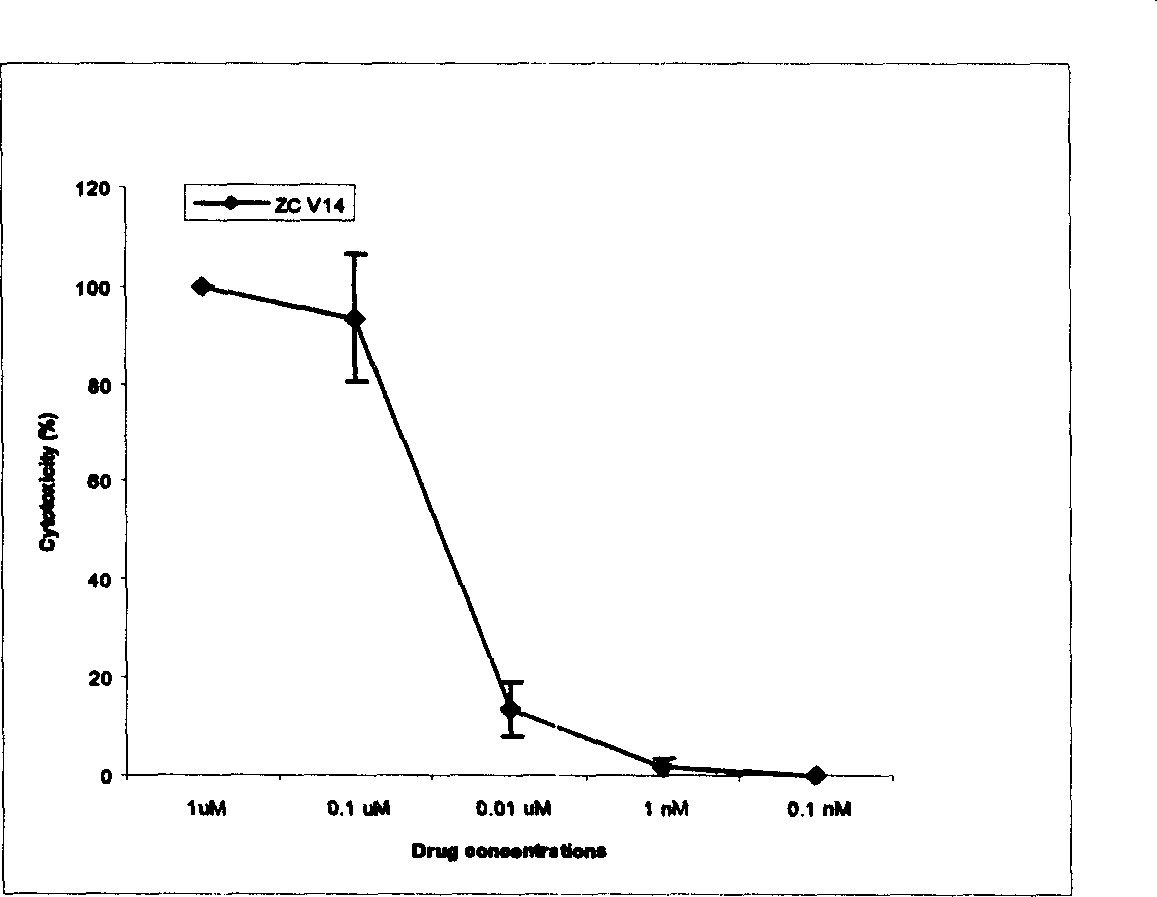
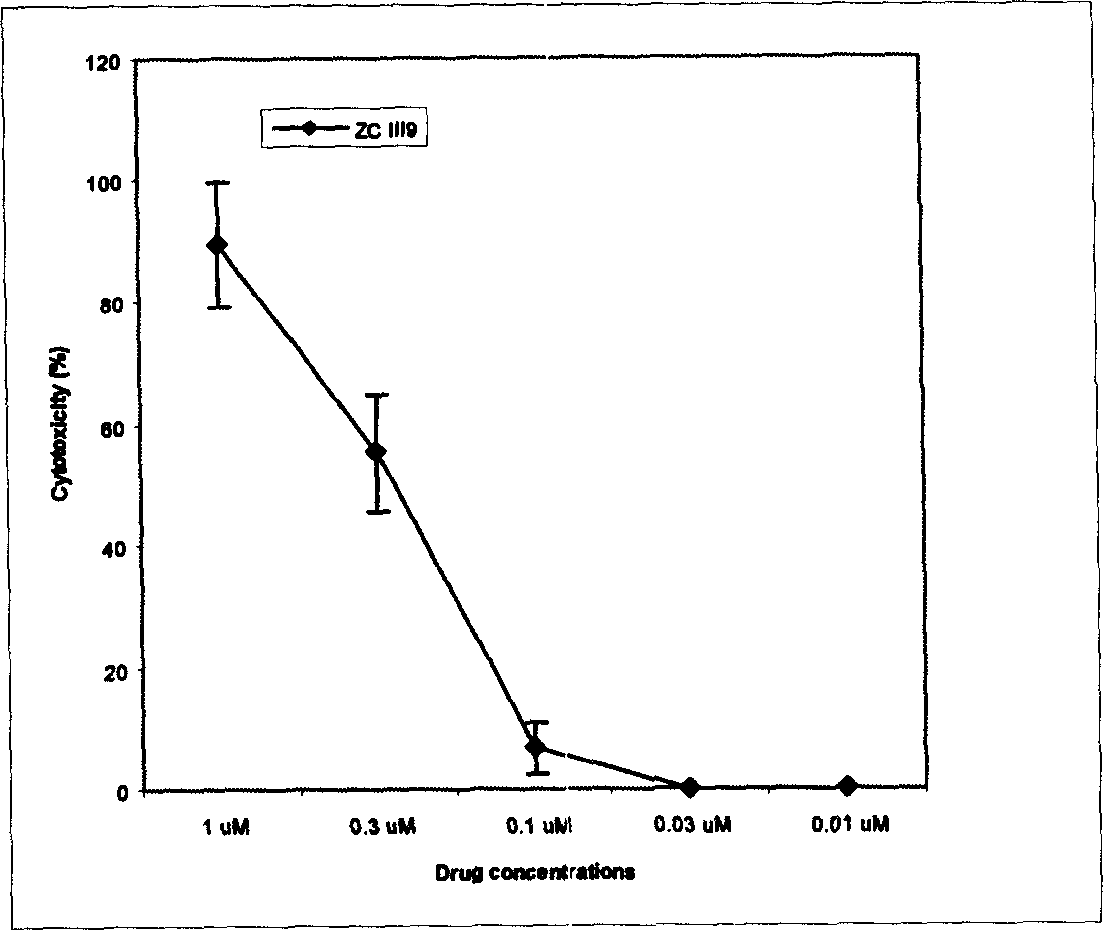
Image
Examples
Embodiment 1
[0081] 1) oleanolic acid-2-bromoethyl ester (1a 1 )
[0082] Suspend OLA (1.0g, 2.19mmol) in 15ml of acetone, add triethylamine (2ml) to dissolve completely, add 1,2-dibromoethane (1.0ml, 11.5mmol) under stirring at room temperature, stir and reflux for 6 hours , filtered off the insoluble matter, concentrated the filtrate, and column chromatography [ethyl acetate:petroleum ether (60~90°C)=1:4(V:V)] gave a white solid 0.80g, yield 65%, mp: 150 ~152°C.
[0083] In the same way, other intermediates such as 1a can be prepared from OLA and other dibromoalkanes, dibromoalkenes, and dibromoalkynes.
[0084] 2) oleanolic acid-2-nitrooxyethyl ester (I 1 )
[0085] 1a 1 (0.4g, 0.71mmol) was dissolved in a mixed solvent of acetonitrile and tetrahydrofuran, silver nitrate (177mg, 1.06mmol) was added, and the light was refluxed for 6 hours, the yellow silver bromide precipitate was filtered off, and the filtrate was concentrated to obtain a light yellow oil The product was subjected...
Embodiment 2
[0092] 1) 4-bromomethylphenyl 3-trifluoroacetoxy oleanolic acid ester (1b 1 )
[0093] OLA (456mg, 1mmol) was suspended in toluene, added trifluoroacetic anhydride (0.8ml, 4mmol), stirred at room temperature for 10 minutes, then added p-hydroxybenzyl bromide (238mg, 1.4mmol), stirred and refluxed for 6 hours, cooled to room temperature, 10 % sodium bicarbonate was washed to neutrality, the organic layer was washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, filtered, and column chromatography [ethyl acetate: petroleum ether (60-90° C.) = 1: 4 (V: V )] to obtain light yellow oil, yield 67.9%.
[0094] 2) 3-trifluoroacetoxy oleanolic acid 4-nitrooxymethylphenyl ester (I 8 )
[0095] 1b 1 (310mg, 0.51mmol) was dissolved in a mixed solvent of acetonitrile and tetrahydrofuran, silver nitrate (127mg, 0.76mmol) was added, and the reaction was stirred under reflux in the dark for 4 hours, and the silver bromide precipitate was filtered off, an...
Embodiment 3
[0102] 1) 4-Hydroxy-3-methoxyphenylacrylate-3-bromopropyl ester (2a 1 )
[0103] Ferulic acid (5g, 25.8mmol) was dissolved in 50ml of acetone, 1,3-dibromopropane (20.2g, 100mmol), 10ml of triethylamine were added, and the reaction was heated at an external temperature of 50°C for 4 hours, cooled to room temperature, and A large amount of white solid precipitated, filtered, the filtrate was concentrated, and column chromatography [ethyl acetate:petroleum ether (60-90°C)=1:4 (V:V)] gave 5.53 g of needle-like solid. Yield: 68%, mp: 100-102°C.
[0104] In the same way, other intermediates such as 2a can be prepared from ferulic acid, vanillic acid, p-hydroxycinnamic acid and other dibromoalkanes, dibromoalkenes, and dibromoalkynes.
[0105] 2) 3-O-trifluoroacetyl-oleanolic acid-2-methoxy-4-[2-(3-bromo-propoxycarbonyl)-vinyl]-phenyl ester (2b 1 ) OLA (1.14g, 2.5mmol) was suspended in toluene, trifluoroacetic anhydride (2ml, 14.85mmol) was added under stirring, the reaction solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com