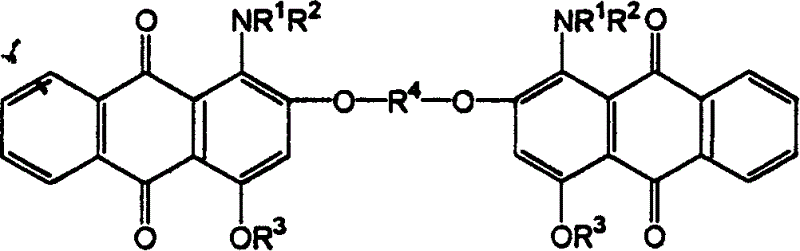
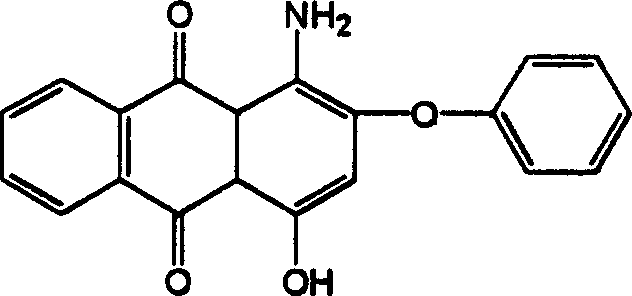
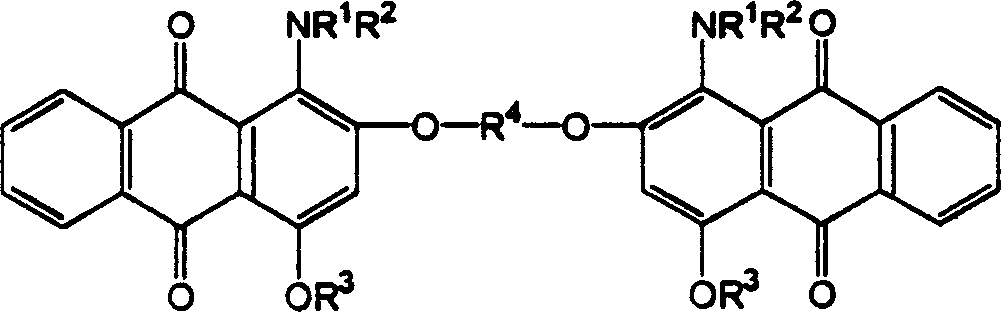
Red anthraquinone dispersion dye
A disperse dye and red technology, applied in the direction of hydroxyanthraquinone dyes, etc., can solve the problems of dye post-processing troubles, increase dye cost, increase reaction steps, etc., and achieve the effect of novel structure, less reaction steps, and good sublimation fastness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 100 ml three-necked flask, add 65 ml of dimethyl sulfoxide solvent, 2.28 g of bisphenol A and 2 g of sodium hydroxide, stir and heat the bisphenol A until it dissolves. Then, add 7.36 grams of 1-amino-2-bromo-4-hydroxyanthraquinone, raise the temperature to 90-150°C, and react for 6-10 hours. After the reaction, cool, filter, wash the product until neutral, dry, and prepare Obtain red anthraquinone disperse dye products.
[0024] The structure of the product was determined and confirmed by mass spectrometry (mass spectrometer: HP 1100LC-MSD) and nuclear magnetic resonance (nuclear magnetic resonance instrument: VarianINOVA400M NMR):
[0025] After the synthesized product is dissolved in chloroform, do APCl mass spectrum, its molecular ion peak is: e / m=702
[0026] H a
H b
H c
H d
H e
H f
H g
δ
(ppm)
6.46(2H)
8.37(2H)
7.77(4H)
8.33(2H)
7.11(2H)
7.36(2H)
1.76(2...
Embodiment 2
[0031] In a 100 ml three-neck flask, add 80 ml of mixed xylene solvent, 1.1 g of hydroquinone, and 2 g of sodium hydroxide, and stir at room temperature until the hydroquinone is dissolved. Then, add 7.50 grams of 1-amino-2-bromo-4-hydroxyanthraquinone, raise the temperature to 90-150°C, and react for 6-10 hours. After the reaction, cool, filter, wash with water until neutral, and dry to obtain Red anthraquinone disperse dye.
[0032] Use above-mentioned mass spectrometer and nuclear magnetic resonance instrument to measure, confirm that its structure is:
[0033]
Embodiment 3
[0035] In a 100 ml three-necked flask, add 2.50 g of bisphenol S, 70 ml of mixed dichlorobenzene solvent and 3 g of potassium hydroxide, and heat under stirring until the bisphenol S dissolves. Then, add 7.65 grams of 1-amino-2-bromo-4-hydroxyanthraquinone, raise the temperature to 90-150°C, and react for 6-10 hours. After the reaction, cool, filter, wash with water until neutral, and dry to obtain Red anthraquinone disperse dye.
[0036] Equally, measure with above-mentioned mass spectrometer and nuclear magnetic resonance instrument, confirm that its structure is:
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com