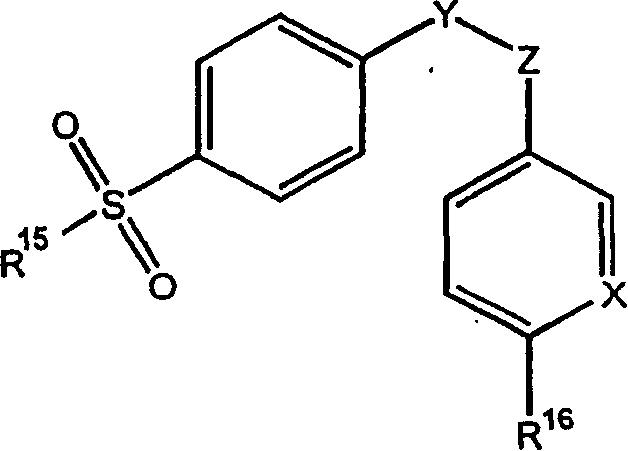
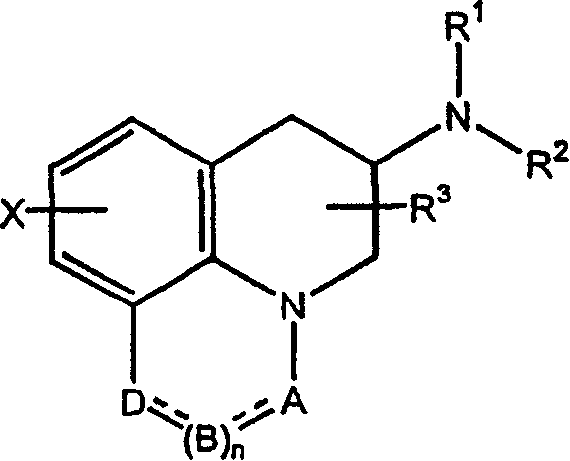
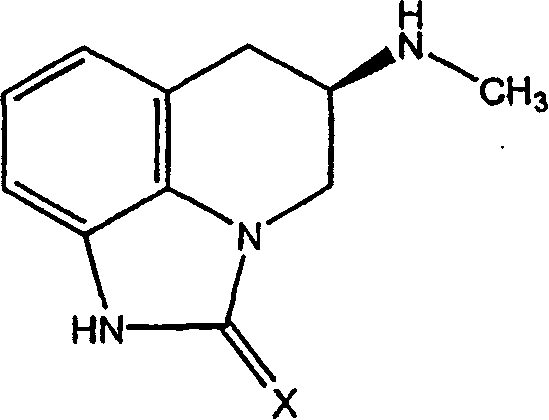
Pharmaceutical dosage form for mucosal delivery
The technology of one drug, all drugs, applied in the direction of drug combination, drug delivery, sugar-coated pills, etc., can solve the problems of not providing the route of administration or dosage form characteristics, and achieve the effect of enhancing the appearance, improving mucoadhesiveness, and improving sensory quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Tablets for sublingual administration of the following compositions are prepared:
[0145] Compound Z 1.11%
[0146] Avicel TM PH-101 (microcrystalline cellulose) 46.71%
[0147] Colorcon Starch 1500 (pregelatinized starch) 44.00%
[0148] Croscarmellose Sodium NF 5.00%
[0149] Colloidal Silica NF 0.50%
[0150] Cinnamon flavoring 0.14%
[0151] Mint flavoring 0.04%
[0152] Colorant (Cherry #1632, Crompton & Knowles) 0.50%
[0153] Magnesium Stearate 2.00%
[0154] Place the pregelatinized starch and colorant in a high shear mixer and mix for 2 minutes or until well combined. Then, the following components were added in portions to the mixture prepared in the high-shear mixer: compound Z; microcrystalline cellulose, colloidal silicon dioxide, and croscarmellose sodium. Mixing was continued for an additional 2 minutes in the high shear mixer. If the colorant is not sufficiently dispersed throughout the mixture, continue mixing for another 1 minute until a go...
Embodiment 2
[0160] The sublingual tablets prepared in Example 1 were coated with gellan gum according to the method described below.
[0161] A coating solution of the following composition was prepared:
[0162] Gellan gum (Kelcogel TM ) 2.00%
[0163] Sodium citrate 0.13%
[0164] Propylene Glycol 0.40%
[0165] Lecithin 0.20%
[0166] Deionized water 97.27%
[0167] Deionized water was heated to 70 °C. Add the other ingredients, stirring as you add until all ingredients are evenly dispersed. The resulting coating solution containing 2.73% solids was maintained at 70°C during stirring and subsequent spraying steps.
[0168] Put the tablet of Example 1, 700 g in total, into a 12-inch (about 300 mm) coating pan, and preheat to a tablet bed temperature of 60° C. The coating solution is sprayed onto the tablet under the following conditions:
[0169] Outlet air temperature 50-60°C
[0170] The rotation speed of the pot is 16rpm
[0171] Air flow 30-35cfm(0.84-0.98m 3 / minute)
...
Embodiment 3
[0176] Sublingual tablets of the following compositions are prepared:
[0177] Compound Z 1.05%
[0178] Mannitol Granules 70.00%
[0179] Sorbitol 16.57%
[0180] Hypromellose, LH-11 type 7.00%
[0181] Xanthan Gum 2.50%
[0182] Colloidal Silica NF 0.50%
[0183] Cinnamon flavoring 0.14%
[0184] Mint flavoring 0.04%
[0185] Colorant (Cherry #1632, Crompton & Knowles) 0.20%
[0186] Magnesium Stearate 2.00%
[0187] The mannitol and colorant were placed in a high shear mixer and mixed for 2 minutes or until uniformly mixed. Then, the following components were added in portions to the resulting mixture in the high shear mixer: compound Z, sorbitol, hypromellose, xanthan gum, colloidal silicon dioxide. Mixing was continued for an additional 2 minutes in the high shear mixer. If the colorant is not sufficiently dispersed throughout the mixture, continue mixing for another 1 minute until a good dispersion of the colorant is observed. A small portion of the mixture is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
| Sheet weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com