Novel dibenzocyclooctenes lignan and its preparation method and use
A technology of biphenyl cyclooctene lignan and medicinal plants, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
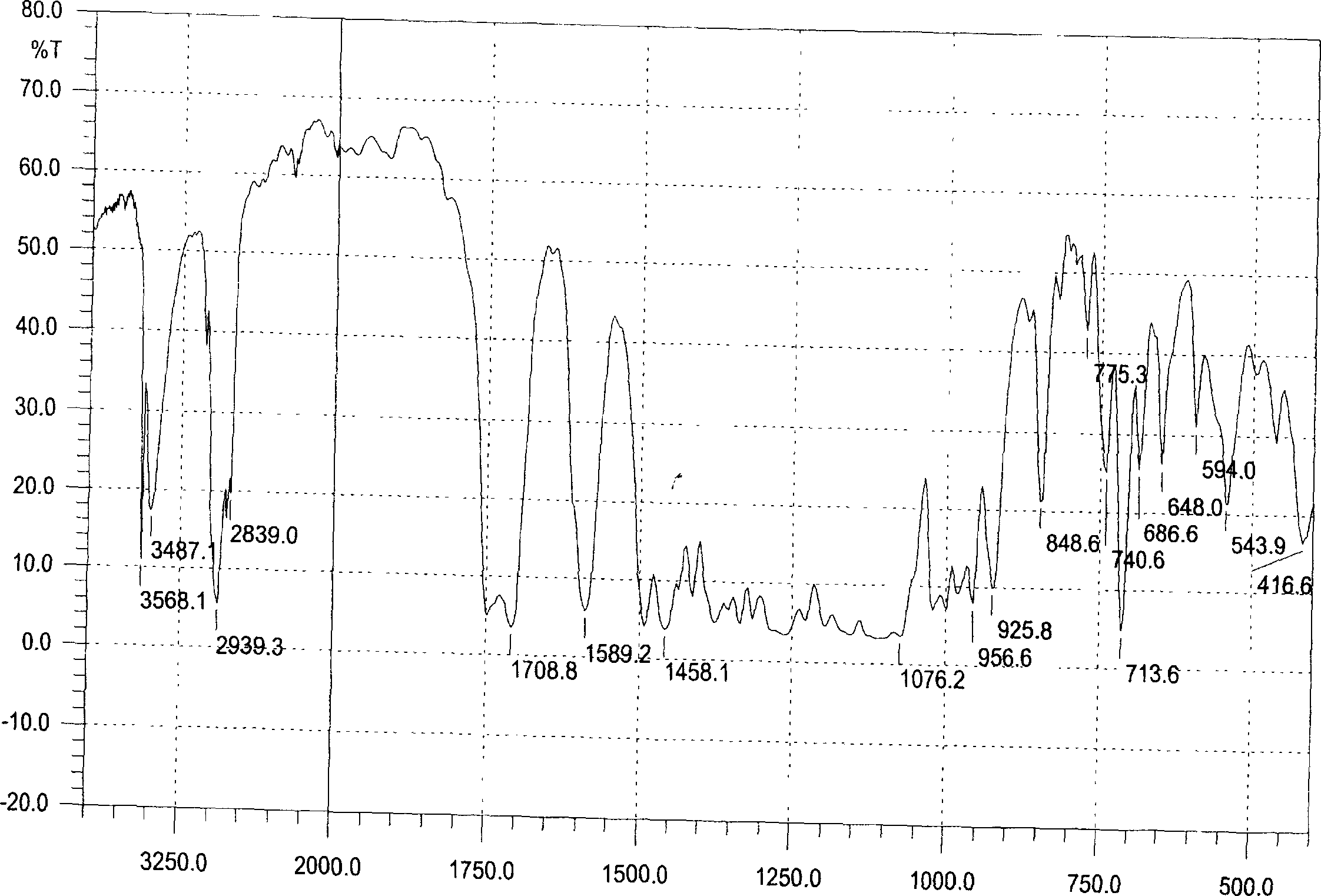
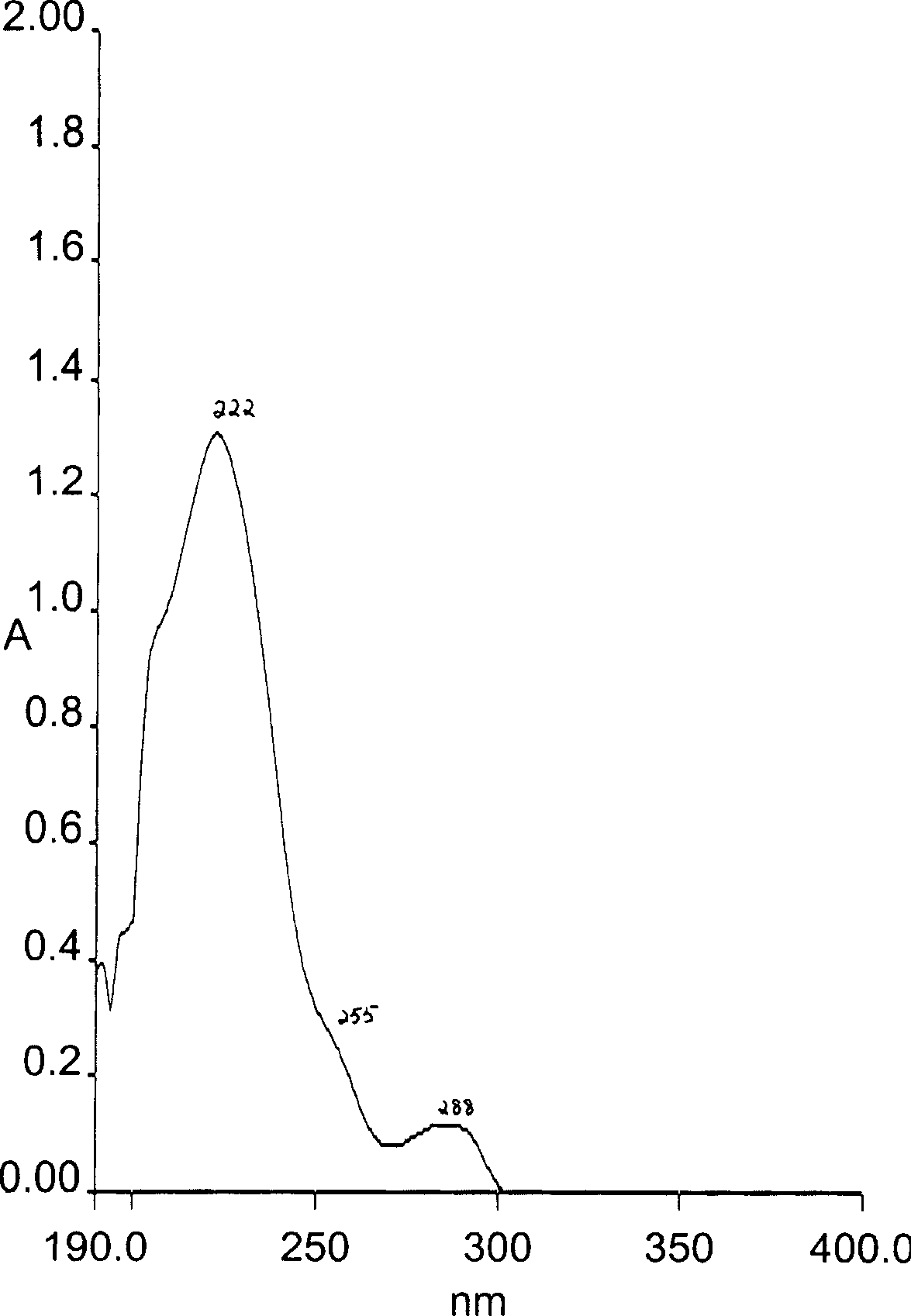
[0032] Embodiment 1: the preparation of formula I compound
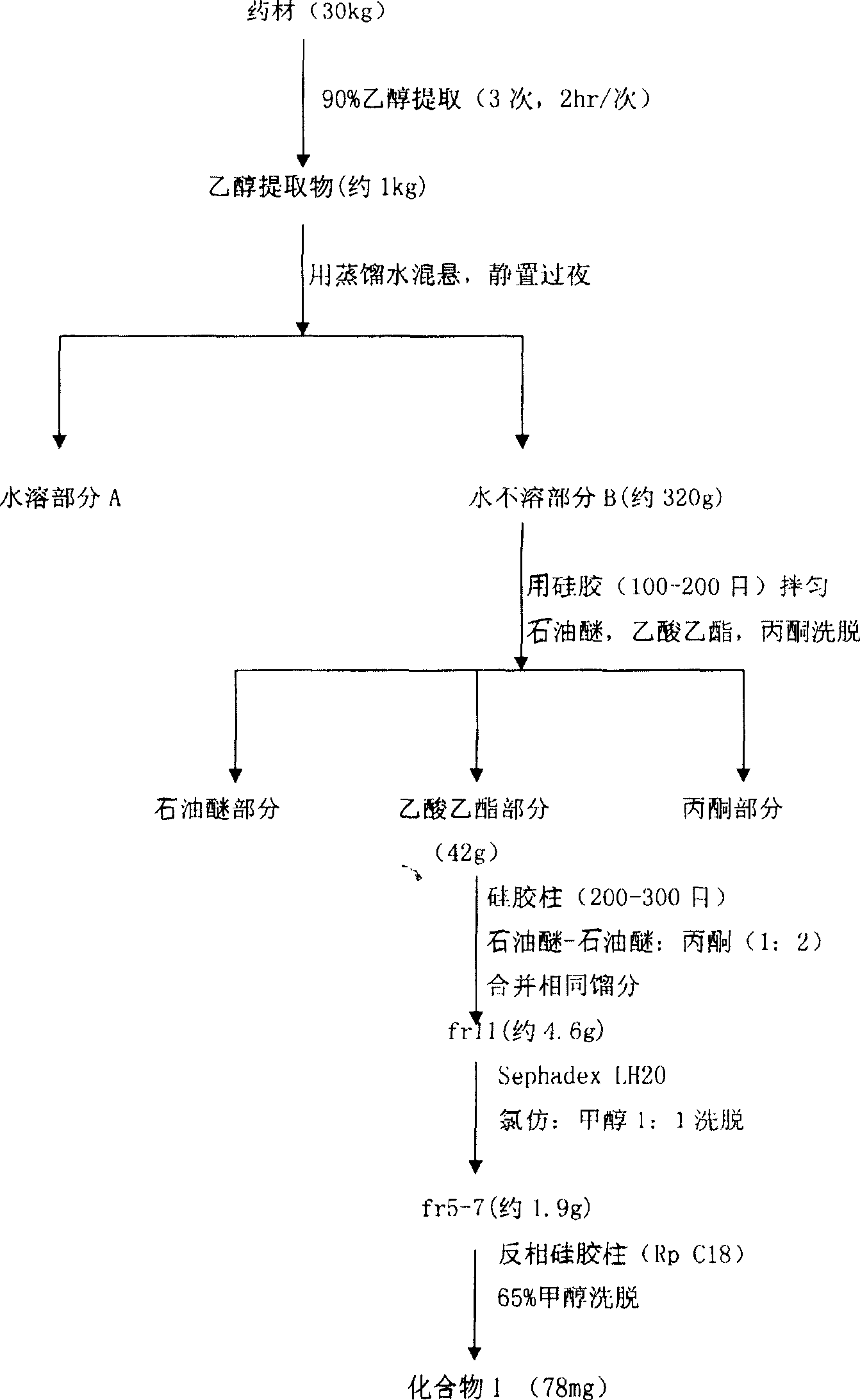
[0033] Take Tiezandra propinqua (Wall.) Baill. 30Kg dry root and stem as raw material, reflux extraction with 95% ethanol three times, each time for 2 hours, concentrate to extract, suspend with appropriate amount of distilled water, let stand overnight, divide The layers yielded a water-insoluble fraction A and a water-soluble fraction B. The water-insoluble part was dissolved with an appropriate amount of ethanol, mixed with crude silica gel (100 mesh), eluted successively with petroleum ether, chloroform, ethyl acetate, and acetone, and then concentrated to extract. Partial extract of ethyl acetate (42g) was separated by silica gel (200-300 mesh) column chromatography, and 15 fractions were obtained by gradient elution with different ratios of petroleum ether to petroleum ether-acetone (1:2) successively. Fraction 11 After separation by Sephadex LH-20 chromatographic column with chloroform:methanol (1:1) as eluen...
Embodiment 3
[0034] Embodiment 3: the in vitro tumor inhibition test of formula I compound:
[0035] 1. Materials: Human acute promyelocytic leukemia cell line (HL-60), human liver cancer cell line (HepG2), and human oral squamous cell carcinoma cell line (KB) were purchased from ATCC, USA. Medium DMEM and fetal bovine serum were purchased from Gibico. MTT (3-(4,5-Dimethyiazol-2,5-diphenyl tetrazolium bromide) was purchased from Sigma.
[0036] 2. Tumor cell culture: HL-60, HepG2 and KB cells were respectively cultured in DMEM (containing 100u / ml Pemcillin and 100ug / ml Streptomycin) medium containing 10% FBS, collected well-grown cells and taken in the logarithmic growth phase Cell culture medium was prepared at a density of 1×10 5 The cell suspension of each cell / ml was uniformly inoculated in a 96-well culture plate, 100ul per well (that is, the number of cells per well was 10 4 pcs) at 5% CO 2 , 95% air, cultured in a cell incubator at 37°C for 24 hours. After the cells were completel...
Embodiment 4
[0050] Preparation of capsules
[0051] Formula I compound 20g
[0052] Lactose 80g
[0053] Magnesium Stearate 1.0%
[0054] 3% povidone ethanol solution appropriate amount
[0055] A total of 1000 capsules were made
[0056] Take prescription amount of compound of formula I and the prescription amount of lactose, mix evenly, add 3% povidone ethanol solution to make soft material, pass through 18 mesh sieve to make granules, dry at 50°C for 30-45 minutes, granulate, add magnesium stearate, mix Evenly, fill in No. 1 capsule. Each capsule contains 20mg of the compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com