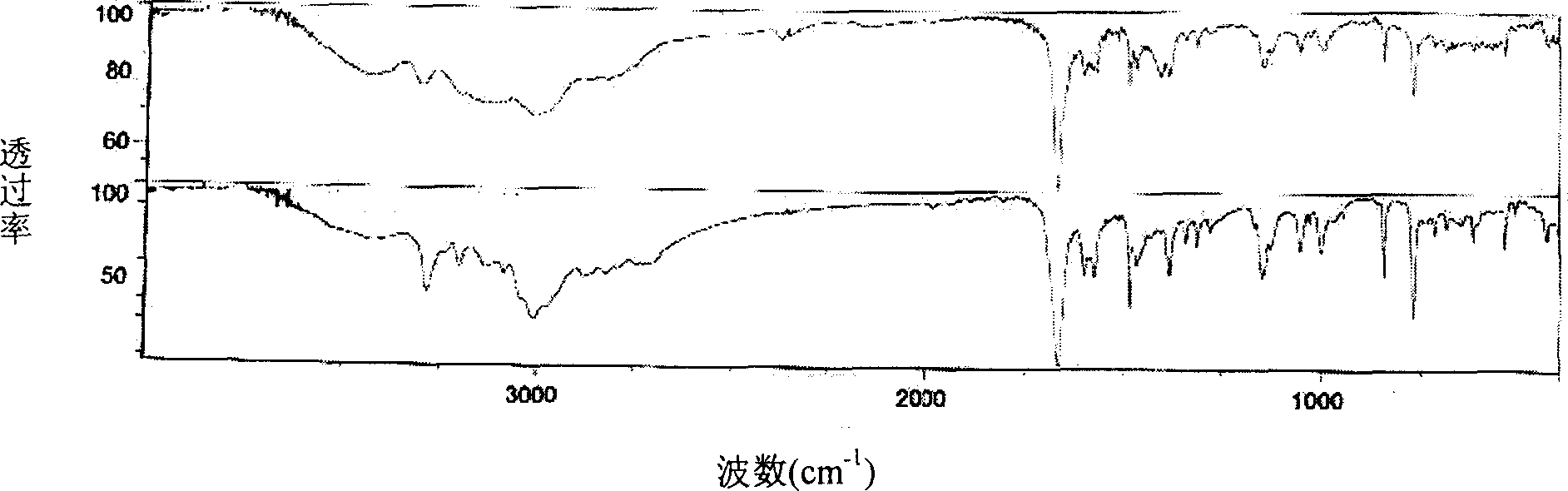
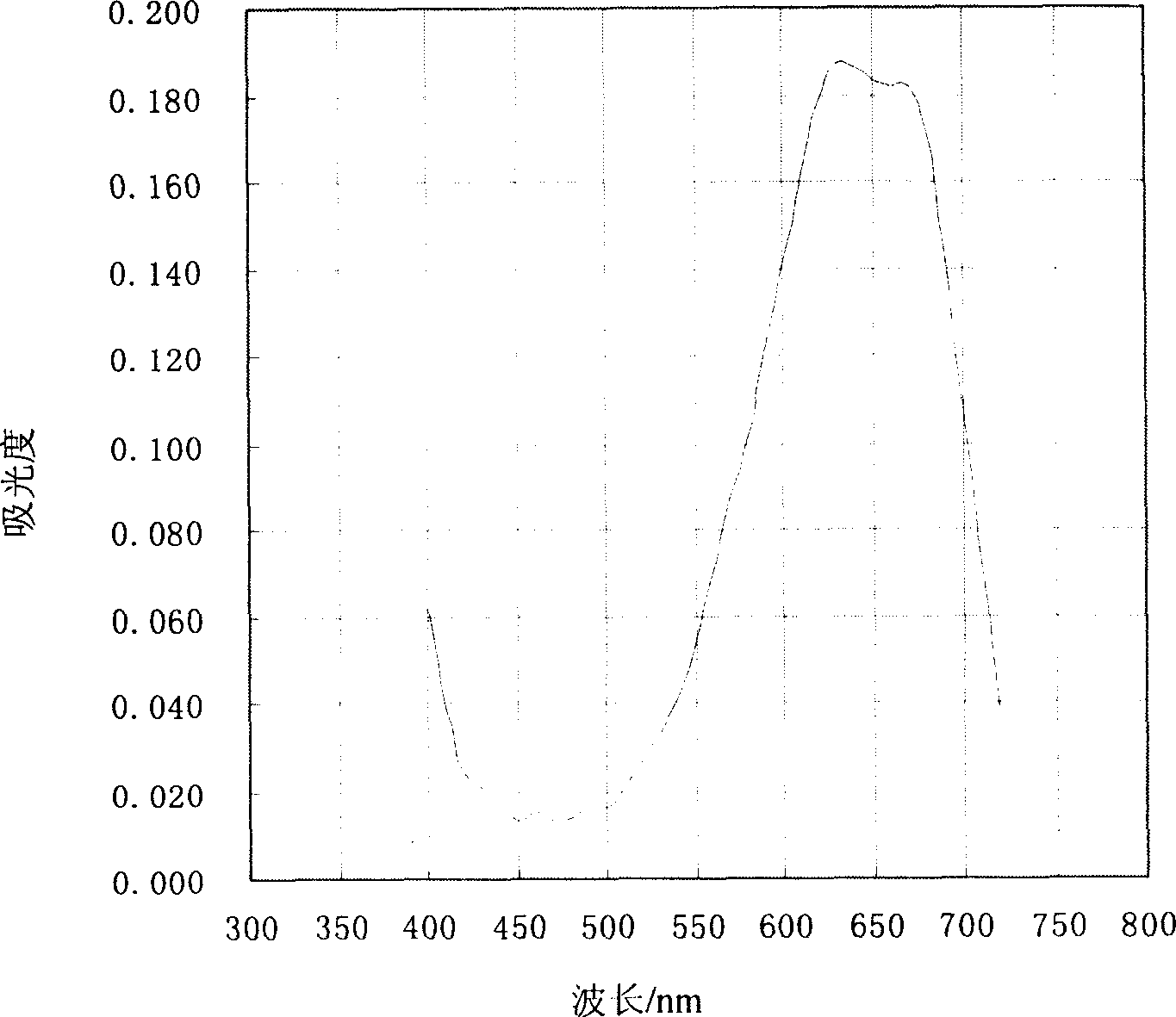
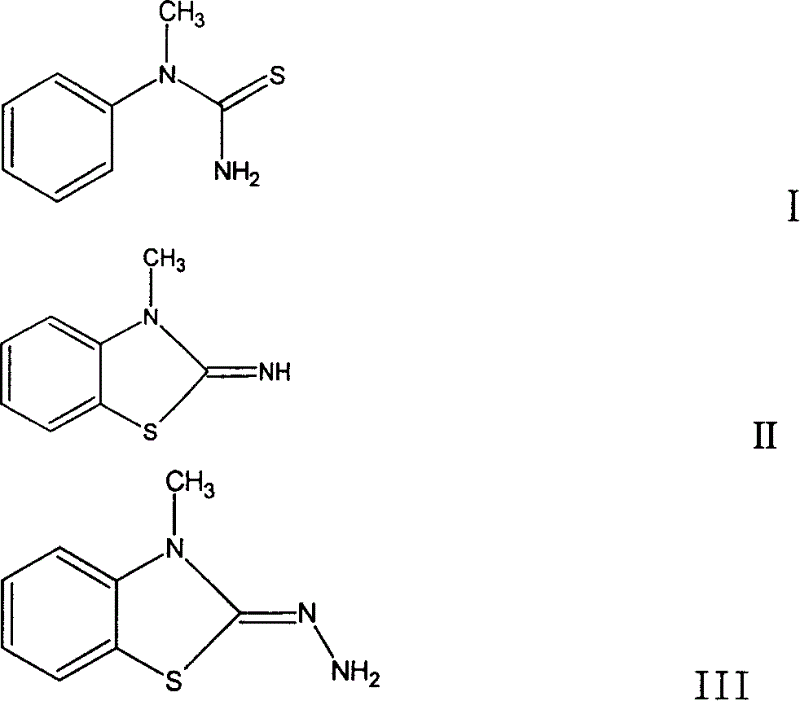
Method for preparing 3-methyl-2-benzothiazolinone hydrazone and its hydrochloride
A technology of benzothiazolinone hydrazone and thiazole hydrobromide, which is applied in the field of preparation of 3-methyl-2-benzothiazolinone hydrazone and its hydrochloride, and can solve the problem of lengthening the synthesis cycle and complicated synthesis steps , high cost and price issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0069] Under reflux and stirring, to a solution of 19.4 g (0.2 mol) of potassium thiocyanate dissolved in 16 mL of distilled water, a mixture of 10.72 g (0.1 mol) of N-methylaniline and 10 mL (0.12 mol) of d1.19 hydrochloric acid was added, and the system Immediately turn pink to bright yellow turbid, then become thick lemon yellow turbid, with oil droplets visible on the liquid surface. Appropriately increase the stirring speed, control the system temperature at 82°C, stop heating after reflux stirring for 4hrs, cool the system rapidly under high-speed stirring, and precipitate yellow loose crystals. The crude product was filtered out, washed with distilled water and dried, and soaked with 10 mL of 95% ethanol to obtain 12.5 g of the crude product. The insoluble solid was recrystallized with 150 mL of hot water, and 7.15 g of small white needle-like crystals were obtained, with a total yield of 43.0%.
[0070] After the crude product was dried, it was dissolved in chloroform...
example 2
[0073] Under reflux and stirring, to a solution of 19.4 g (0.2 mol) of potassium thiocyanate dissolved in 16 mL of distilled water, a mixture of 10.72 g (0.1 mol) of N-methylaniline and 10 mL (0.12 mol) of d1.19 hydrochloric acid was added, and the system Immediately turn pink to bright yellow turbid, then become thick lemon yellow turbid, with oil droplets visible on the liquid surface. Appropriately increase the stirring speed, control the system temperature at 92°C, stop heating after reflux stirring for 4hrs, cool the system rapidly under high-speed stirring, and precipitate yellow loose crystals. The crude product was filtered out, washed with distilled water and dried, and soaked with 10 mL of 95% ethanol to obtain 12.5 g of the crude product. The insoluble solid was recrystallized with 150 mL of hot water, and 7.56 g of small white needle-like crystals were obtained, with a total yield of 45.5%.
[0074] After the crude product was dried, it was dissolved in chloroform...
example 3
[0077] Under reflux stirring, to the solution of sodium thiocyanate 81g (1.0mol) dissolved in 80mL distilled water, add the mixture of N-methylaniline 53.6g (0.5mol) and d1.19 hydrochloric acid 50mL (0.12mol). Stirring speed, control system temperature at 85°C, stop heating after reflux stirring reaction for 8hrs, rapidly cool the system under high-speed stirring, and precipitate yellow loose crystals. The crude product was filtered out, washed with distilled water and drained, and then recrystallized with 200 mL of 95% ethanol. The yield was 47.2 g, and the total yield was 55.8%.
[0078] After the crude product was dried, it was dissolved in chloroform, and it was analyzed by TLC with 20:10:1 chloroform-petroleum ether-acetic acid solution as developing solvent. The raw material N-methylaniline was used as internal standard, and only one spot was seen in the product, Rf0.288; internal standard Raw material point Rf0.562, the reaction is basically complete.
[0079] Purified...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com