Oxazolidinone derivatives as antimicrobials
A solvate, alkyl technology, applied in the field of substituted phenyl oxazolidinones and their synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194] (S)-N-[[3-[3-fluoro-4-(N-piperazinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide (core I) analog
[0195] A heteroaryl group with a corresponding additional group can be introduced on the ring C nitrogen atom of the compound of formula I as follows:
[0196] General method:
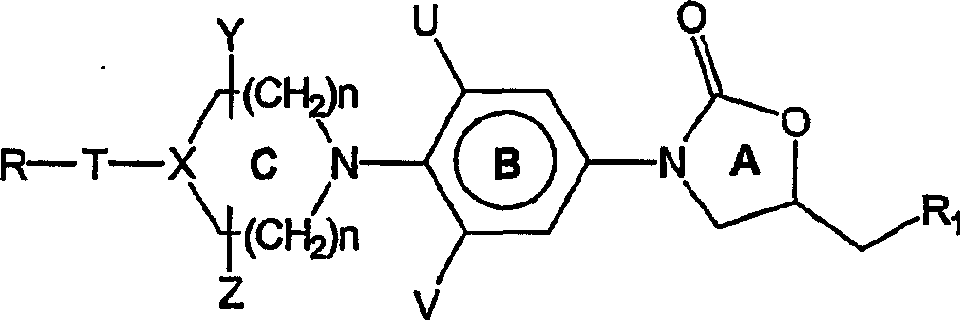
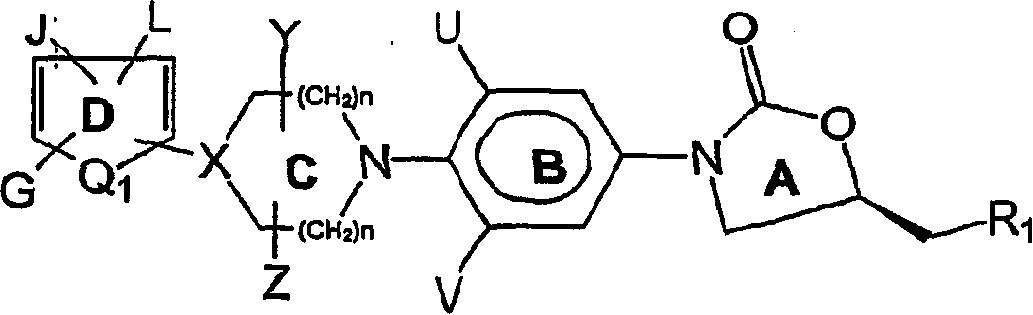
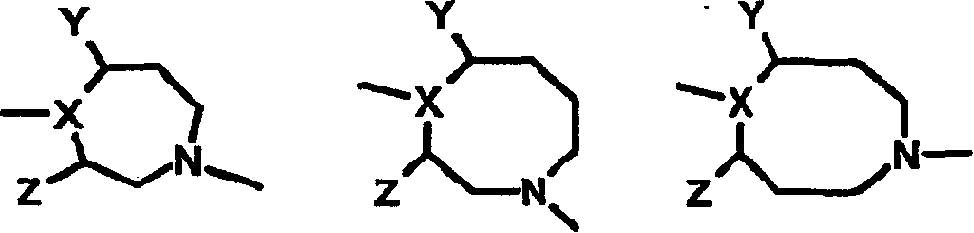
[0197] Amines of formula VI with as suitable leaving groups (such as fluorine, chlorine, bromine, iodine, SCH 3 , SO 2 CH 3 、-SO 2 CF 3 , Tos or OC 6 h 5 etc.) R 12 The heteroaromatic compound of formula VII is reacted as shown in previous reaction scheme I. Q 1 , G, J and L are as defined in Formula II. The reaction is carried out in a suitable solvent such as dimethylformamide, dimethylacetamide, acetonitrile, dimethyl sulfoxide or ethylene glycol at a suitable temperature from -70°C to 180°C to give the compound of formula II.
[0198] In some cases, the presence of a suitable base such as triethylamine, diisopropylethylamine, potassium carbonate, potassium bicarbonate, dipotassium...
Embodiment 2
[0223] Analogs of (S)-N-[[4-(N-piperazinyl)-phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide (Core II)
[0224] Compound No. 6: (S)-N-[[3-[4-[N-1-(5-nitro-2-thienyl)piperazinyl]-phenyl]-2-oxo-5-oxa Preparation of oxazolidinyl]methyl]acetamide
[0225] Trifluoroacetic acid (S)-N-[[3-[4-(1-piperazinyl)-phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide (1.076mmol) and Acetone and K 2 CO 3 (200 mg) was stirred for 5 minutes, then filtered and concentrated under reduced pressure. The residue was dissolved in DMSO and stirred at room temperature. At room temperature, adding K 2 CO 3 (224mg, 1.61mmol) and 2-bromo-5-nitrothiophene (246mg, 1.18mmol) was stirred overnight. The reaction mixture was quenched with water and extracted with DCM. with anhydrous Na 2 SO 4 The organic layer was dried and concentrated under reduced pressure to obtain a crude product, which was purified by column chromatography. (Silica gel 100-200 mesh) Elution: 1-2% MeOH in DCM gave 75 mg of the title compo...
Embodiment 3
[0229] (S)-N-[[3-[3-fluoro-[4-(1-piperazinyl)-phenyl]-2-oxo-5-oxazolidinyl]-2methyl]-chloropropane Analogs of amides (Core III)
[0230] Compound No. 7: (S)-N-[[3-[3-fluoro[4-[N-1-{4-(5-nitro-2-thienyl)piperazinyl}]-phenyl]- Preparation of 2-oxo-5-oxazolidinyl]methyl]-2-chloro-propionamide
[0231] (S)-N-[[3-fluoro-[4-(1-piperazinyl)-phenyl]-2-oxo-5-oxazolidinyl]methyl]-2-chloro-propionamide (WO 00 / 32599) (0.22 g, 0.454 mmol) was dissolved in acetonitrile. Here, N-ethyldiisopropylamine (0.117 g, 0.9 mmol) and 5-nitro-2-bromo-thiophene (0.13 g, 0.681 mmol) were added and the reaction mixture was heated at 60° C. for 6-8 hours. The reaction mixture was concentrated. The crude compound was purified by column chromatography eluting with 2% methanol in dichloromethane.
[0232]1 HNMR (CDCl 3 ): δppm 8.23(m, 1H, NH), 7.8(d, 1H, Ar-H), 7.47(m, 1H, Ar-H), 6.98(m, 1H, Ar-H), 6.95(m, 1H , Ar-H), 6.06(d, 1H, Ar-H), 4.79(m, 1H, CH), 4.45(m, 1H, CH), 4.0(m, 1H, CH), 3.81(m, 1H, CH)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com