Process for the production of methionine
A technology of methionine and amino group is applied in the field of preparation of methionine crystal, which can solve the problems of low solubility, difficult recovery of biocatalysts, and inability to increase the dissolved accumulation of methionine.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
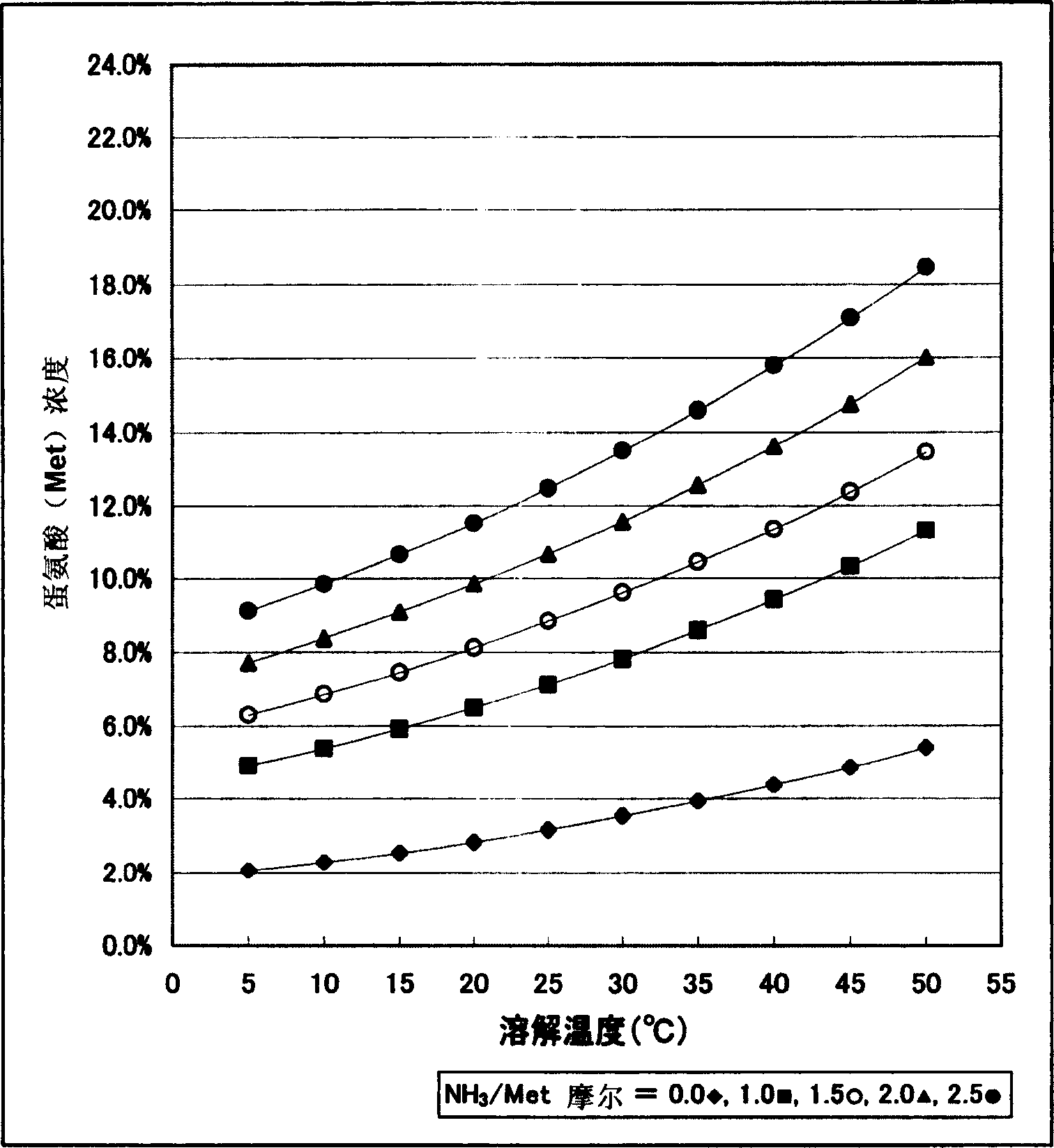
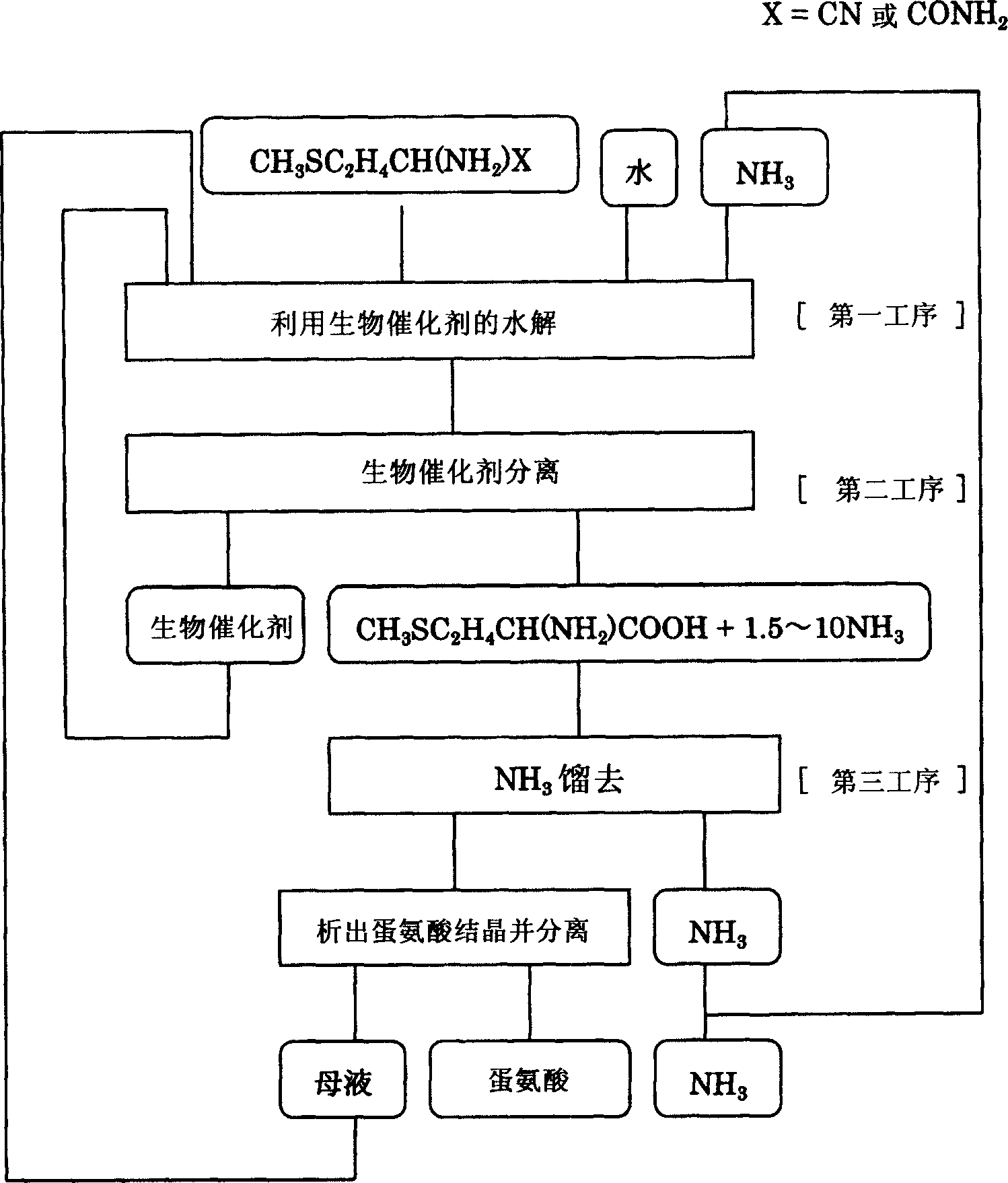
[0024] The preparation method of methionine of the present invention is not particularly limited as long as it has the following steps, that is, (1) the first step: using a biocatalyst with hydrolysis activity in an ammonia solution to decompose a raw material that can be hydrolyzed to methionine Carry out hydrolysis, preferably utilize the biocatalyst that has nitrile hydrolysis activity to hydrolyze raw material 2-amino-4-methylthiobutyronitrile in ammonia solution or utilize the biocatalyst that has amide hydrolysis activity to 2-amino- 4-Methylthiobutyramide is hydrolyzed to be converted into an ammonia solution containing methionine; (2) second process: the ammonia solution containing methionine obtained in the aforementioned first process is separated from the biocatalyst; (3) the third process : Ammonia is distilled off from the methionine-containing ammonia solution separated in the second step, and methionine is crystallized and separated. For example, if figure 2 A...
Embodiment 1
[0036] Hereinafter, although an Example demonstrates in more detail, this invention is not limited to these Examples. Example 1 (Preparation of DL-Methionine by NSSC204 Strain) (Cultivation of NSSC204 Strain)
[0037] Containing 0.5% of yeast extract, 0.5% of glucose, 0.1% of dipotassium hydrogen phosphate, 0.1% of potassium dihydrogen phosphate, 0.1% of common salt, 0.02% of magnesium sulfate heptahydrate, 0.001% of ferrous sulfate and 0.03% of 2-aminobenzonitrile 2ml of the culture medium was placed in a test tube and sterilized at 121°C for 20 minutes. One Arthrobacter strain NSSC204 of Platinum auricularia was implanted in the test tube, and cultured with shaking at 33° C. overnight to prepare a pre-culture. Next, 2.0% of corn steeping liquid (sterilized by filtration), 1.0% of sucrose (sterilized at 121°C for 20 minutes), 0.03% of 2-aminobenzonitrile (sterilized at 121°C for 20 minutes), pH 7.2 (with 2N Caustic soda adjusted) medium 20ml was added to a 100ml Erlenmeyer ...
Embodiment 2
[0040] Embodiment 2 (continuous production of DL-methionine by NSSC204 strain)
[0041] The culture solution of the Arthrobacter NSSC204 strain obtained in Example 1 was centrifuged, washed with ion-exchanged water, and then suspended in an aqueous solution containing 10% (W / W) DL-methionine and 2.28% (W / W) ammonia. In the aqueous solution (pH9.5), the concentration is 2% (W / W) in terms of dry cells. 300 g of this bacterial cell suspension was added to a three-neck flask with a capacity of 500 ml maintained at a temperature of 30° C., and 2-amino-4-methylthiobutyronitrile was continuously added at a rate of 5.5 g per hour while stirring. In addition, bacterial cells were continuously filtered using a microfiltration membrane (microza PMP-003 manufactured by Asahi Kasei), and the reaction filtrate was recovered at a rate of about 54 g per hour. At this time, in order not to reduce the amount of liquid in the reaction vessel, a pump linked to the liquid level sensor was used to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com