Methods and compositions of novel triazine compounds
A compound, trifluoromethyl technology, applied in the field of preparation and use of triazine compounds, can solve problems that are difficult to treat and expensive to treat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
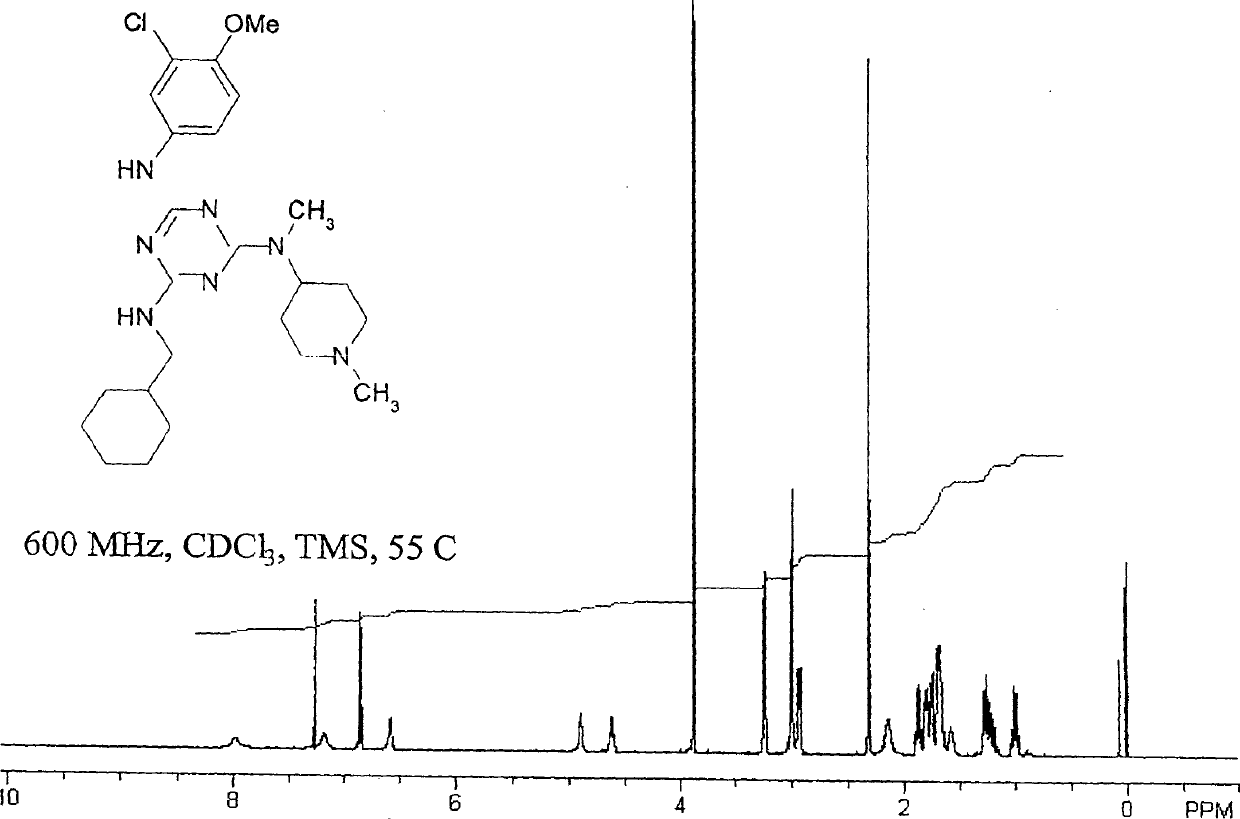
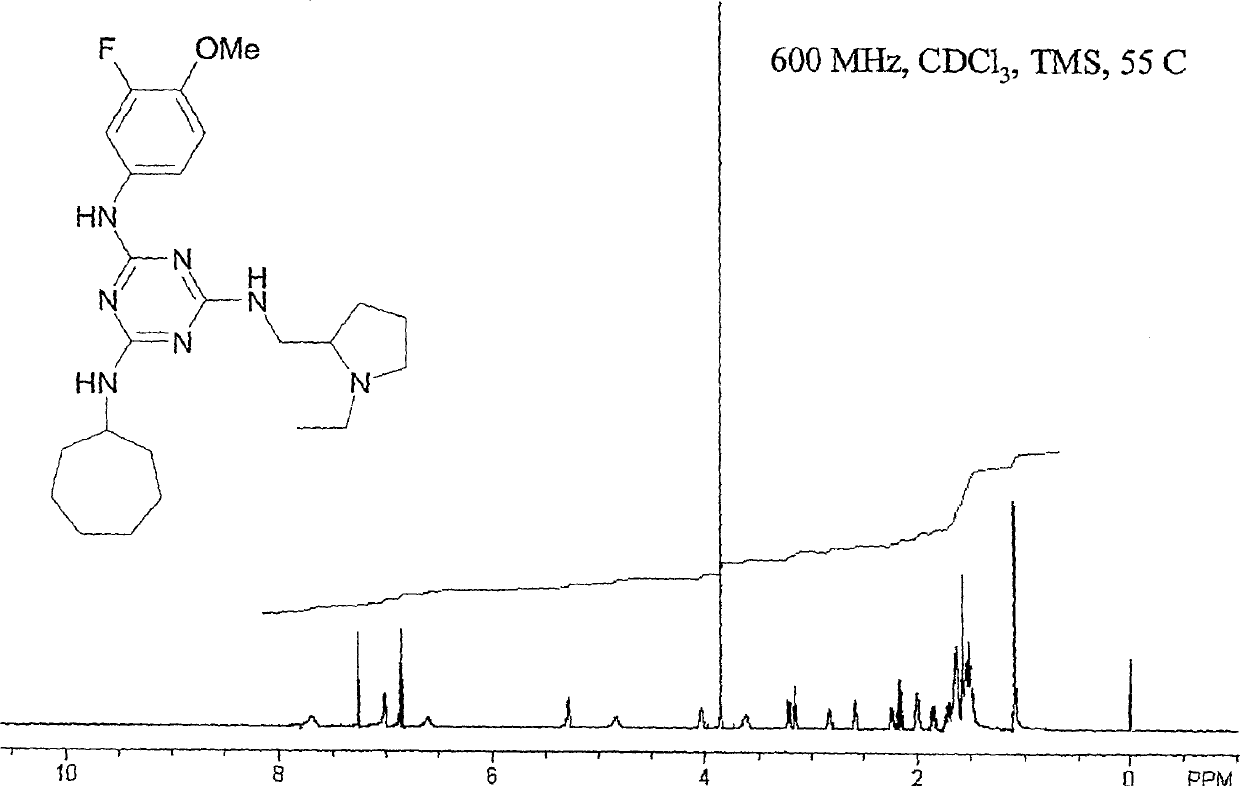
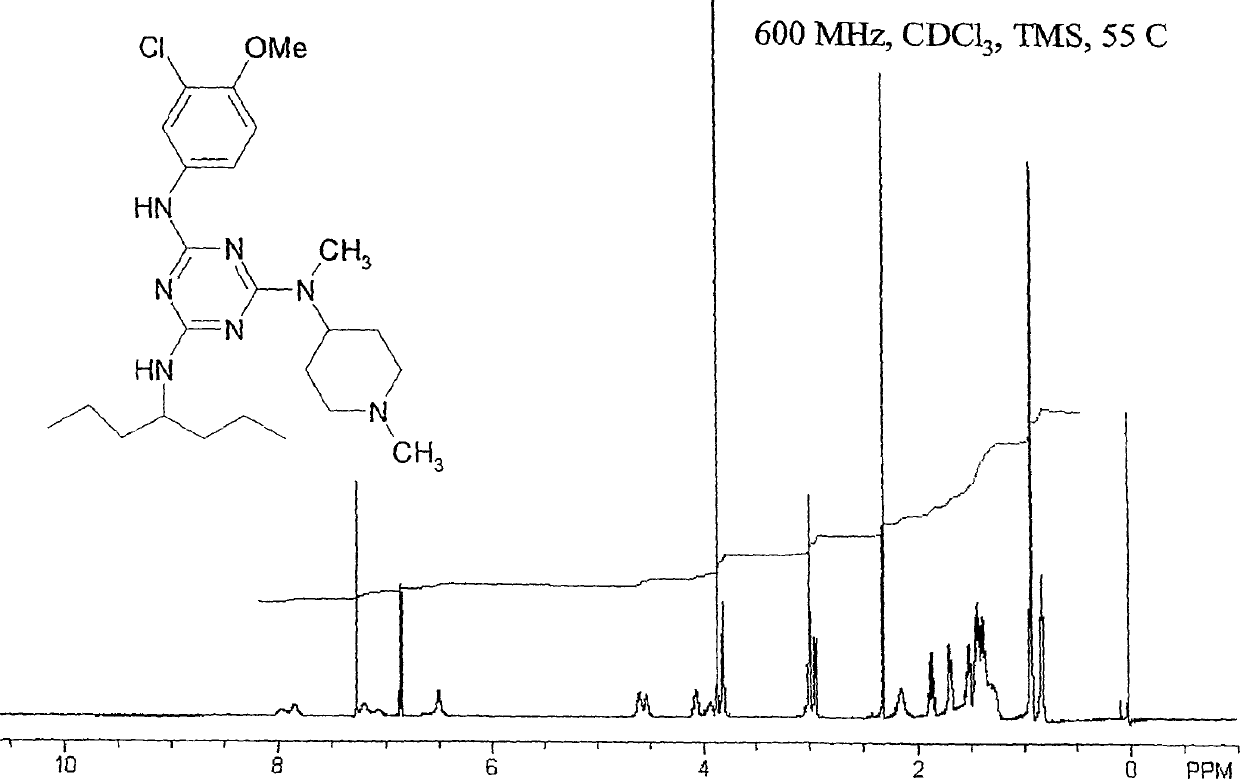
[0125] In this typical embodiment, each side group NR 1 R 2 、NR 3 R 4 and NR 5 R 6 Amino groups when bound to a triazine core can represent primary, secondary or tertiary amines, including cyclic secondary amide substituents (eg, pyrrolidin-N-yl) and other substituents within the range described herein. The compositions of the present invention also include tri(amino) compounds such as compounds prepared as intermediate compounds in the synthesis of the aforementioned tri(amino)triazine compounds or compounds representing partially substituted triazine nuclei. Many synthetic methods of triazine compounds of the present invention generally use cyanuric chloride C 3 N 3 Cl 3 As starting compounds, the present invention thus also includes intermediate species such as bis(amino)chlorotriazine compounds or aminodichlorotriazine compounds as shown below, wherein N A and N B is a substituted amino side group as described above.
[0126]
[0127] Compositions of the prese...
Embodiment 1
[1220] General synthesis, purification, characterization and spectroscopic steps
[1221] General Synthetic Procedure.
[1222] Room temperature is defined as the ambient temperature range, generally between 20-25°C. The ice bath (crushed ice / water) temperature is defined as generally in the range of -5-0°C. The reflux temperature was defined as ±15°C from the boiling point of the main reaction solvent. Overnight is defined as the 8-16 hour time frame. Vacuum filtration (water pump) is defined as the range of 5-15 mmHg. Drying in vacuum is defined as using a high vacuum pump in the range of 0.1-5 mmHg. Neutralization was defined as a typical acid-base neutralization method and was used to determine the pH range of 6-8 using pH-test paper. Saline is defined as saturated aqueous sodium chloride. The nitrogen atmosphere was defined as the normal pressure of nitrogen passing through a Drierite column with a diffuser system. Concentrated ammonium hydroxide is defined as an a...
Embodiment 2
[1238] General method for parallel synthesis
[1239] Examples 3-5 describe the preparation of N 2 , N 4 , N 6 - Synthetic procedure for a "library" of tris(amino)-1,3,5-triazines based on a strategy of changing only one amino side group per synthesis and based on the parent structure 95 shown below, where in Each compound of contains two of the side groups on 95.
[1240]
[1241] 95
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com