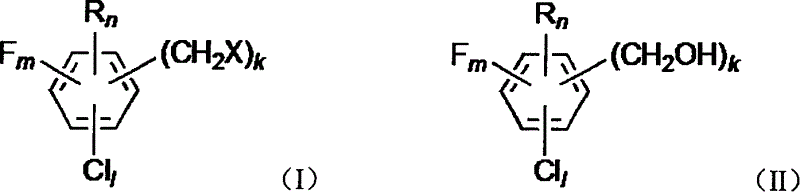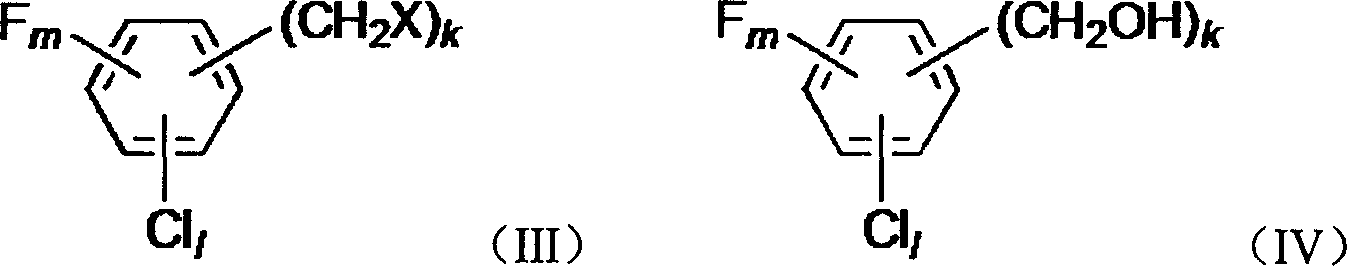Method for producing substituted benzyl alcohol by continuous process and its apparatus
A technology of benzyl alcohol and benzyl halide, which is applied in the production field of replacing benzyl alcohol, can solve the problems of large alkali consumption, and achieve the effect of reducing waste discharge and resource consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
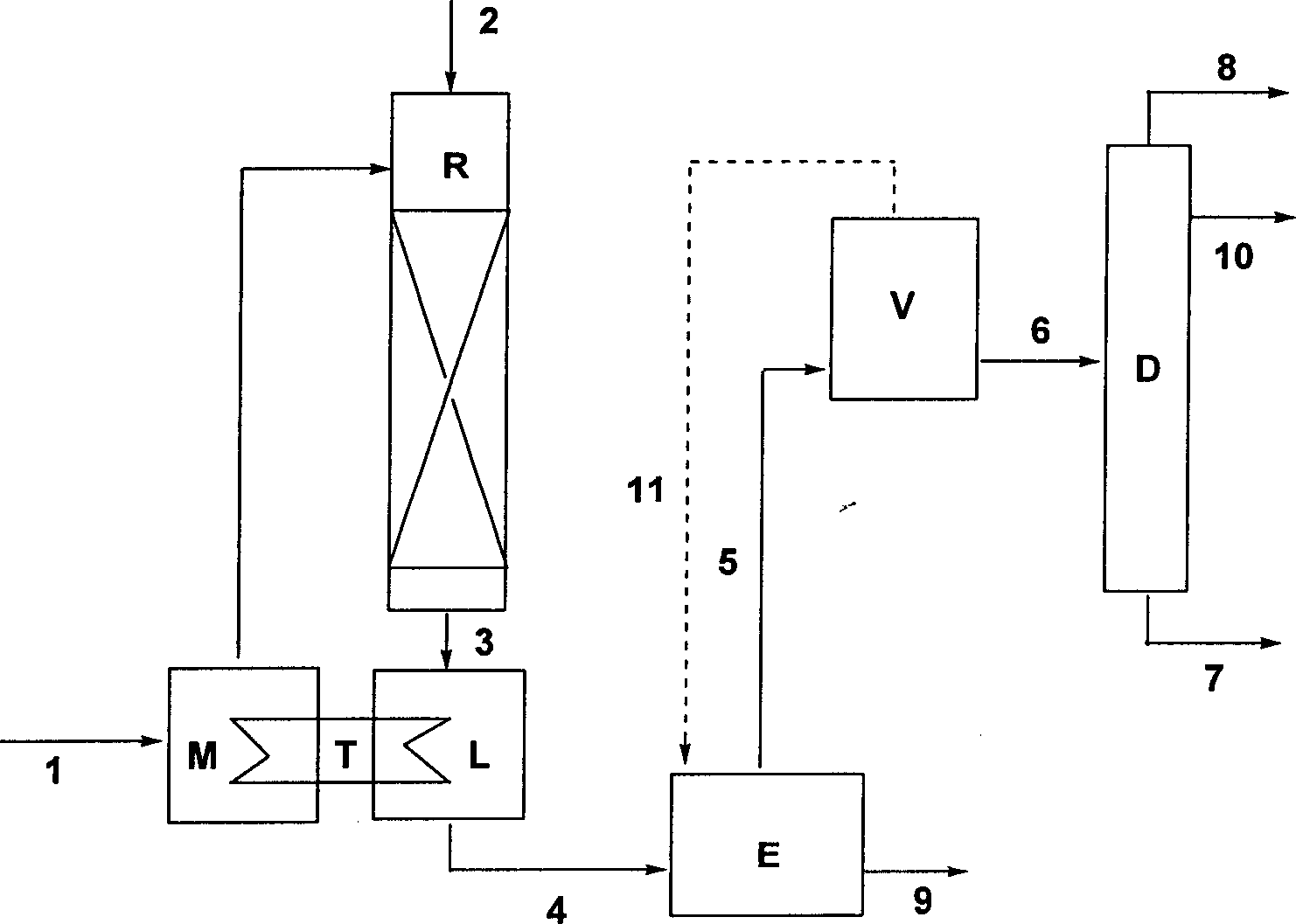
[0050] The raw material substituted benzyl chloride 1 and water are mixed in the mixer M and heated to 80°C, then fed continuously from the top of the reactor R by a pump, and the volume ratio of the substituted benzyl halide to water is 1:15. The temperature in the reactor is controlled at 120-150°C. Reactor R is a tubular constant temperature reactor filled with inert fillers (such as glass beads). The aspect ratio of the reactor is 1:3, and the pressure is controlled at 0.4MPa. The raw material substituted benzyl chloride 1 is evenly sprayed on the filler through the distributor at the top of the reactor R (for evenly distributing the material on the filler), and at the same time, water vapor 2 is introduced from the top of the reactor, and the substituted benzyl chloride 1 passes through reactor. The raw material substituted benzyl chloride 1 is distributed on the inert filler in the reactor in a liquid state to form a trickle bed, which increases the mass transfer area ...
Embodiment 2
[0055] The raw material substituted benzyl bromide 1 and water are mixed in the mixer M and heated to 80°C, then fed continuously from the top of the reactor R by a pump, and the volume ratio of substituted benzyl halide to water is 1:5. The temperature in the reactor is controlled at 160-180°C. Reactor R is a tubular constant temperature reactor filled with inert fillers (such as Raschig rings). The aspect ratio of the reactor is 1:7, and the pressure is controlled at 0.2MPa. The raw material substituted benzyl bromide 1 is evenly sprayed on the filler through the distributor at the top of the reactor R, and at the same time, water vapor 2 is introduced from the top of the reactor, and the substituted benzyl bromide 1 passes through the reactor R in parallel. The raw material substituted benzyl bromide 1 is distributed on the inert filler in the reactor in a liquid state to form a trickle bed, which increases the mass transfer area and improves the mass transfer efficiency; ...
Embodiment 3
[0059] Example 3: Preparation of 2-chlorobenzyl alcohol by hydrolysis of 2-chlorobenzyl chloride
[0060] Referring to the method of Example 1 (unmentioned parameters are the same as Example 1), heat 2-chlorobenzyl chloride and water (volume ratio 1:1) to 80° C., mix well, and pass through a metering pump at a space velocity of 0.2h -1 Entering the trickle bed reactor, the reactor R controls the temperature at 130-135°C and the pressure at 0.15MPa. Reactor R is filled with Raschig rings with a volume of 1L. Water vapor is introduced from the top of the reactor R with a space velocity of 0.6h -1 . The oil phase (that is, the organic phase) after passing through the bed of the reactor R can be determined to contain 86% substituted benzyl alcohol, 4% dibenzyl ether and 10% aqueous hydrochloric acid solution containing 2.6%.
[0061] The reactant is cooled to 30°C through the condenser L, and added to the continuous extraction tank E, and the extraction agent toluene is added a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com