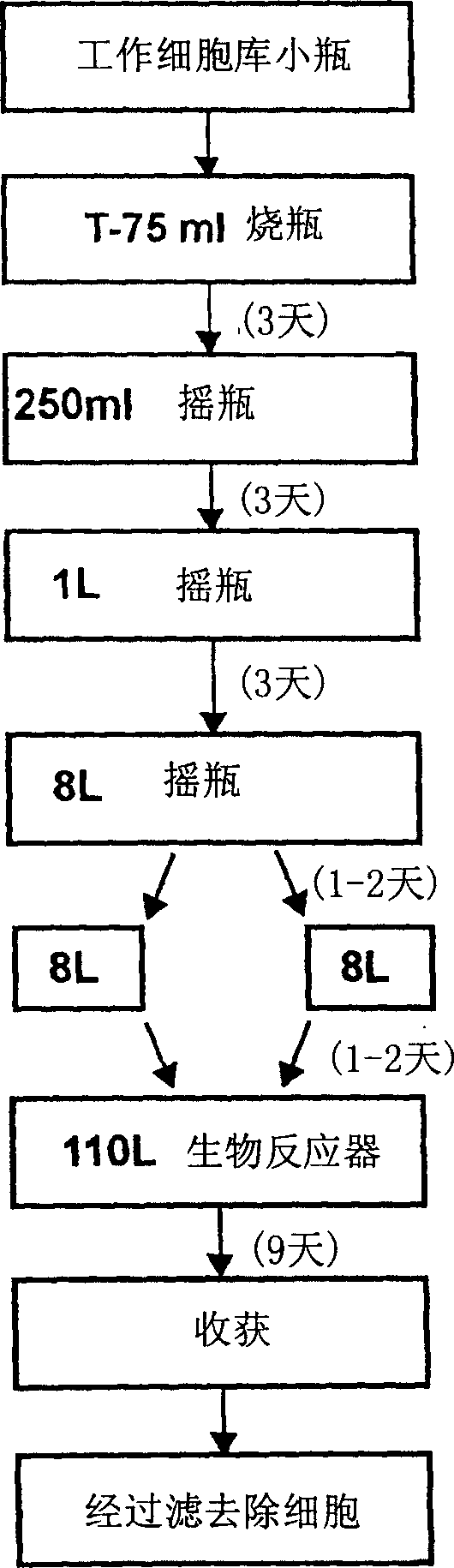
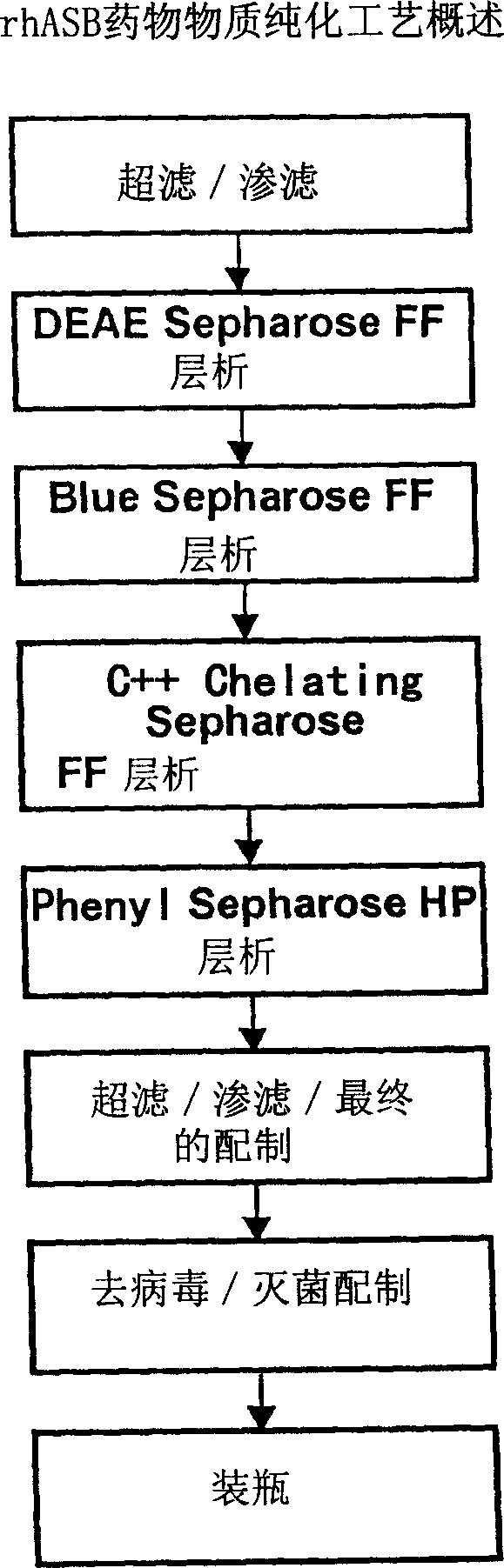
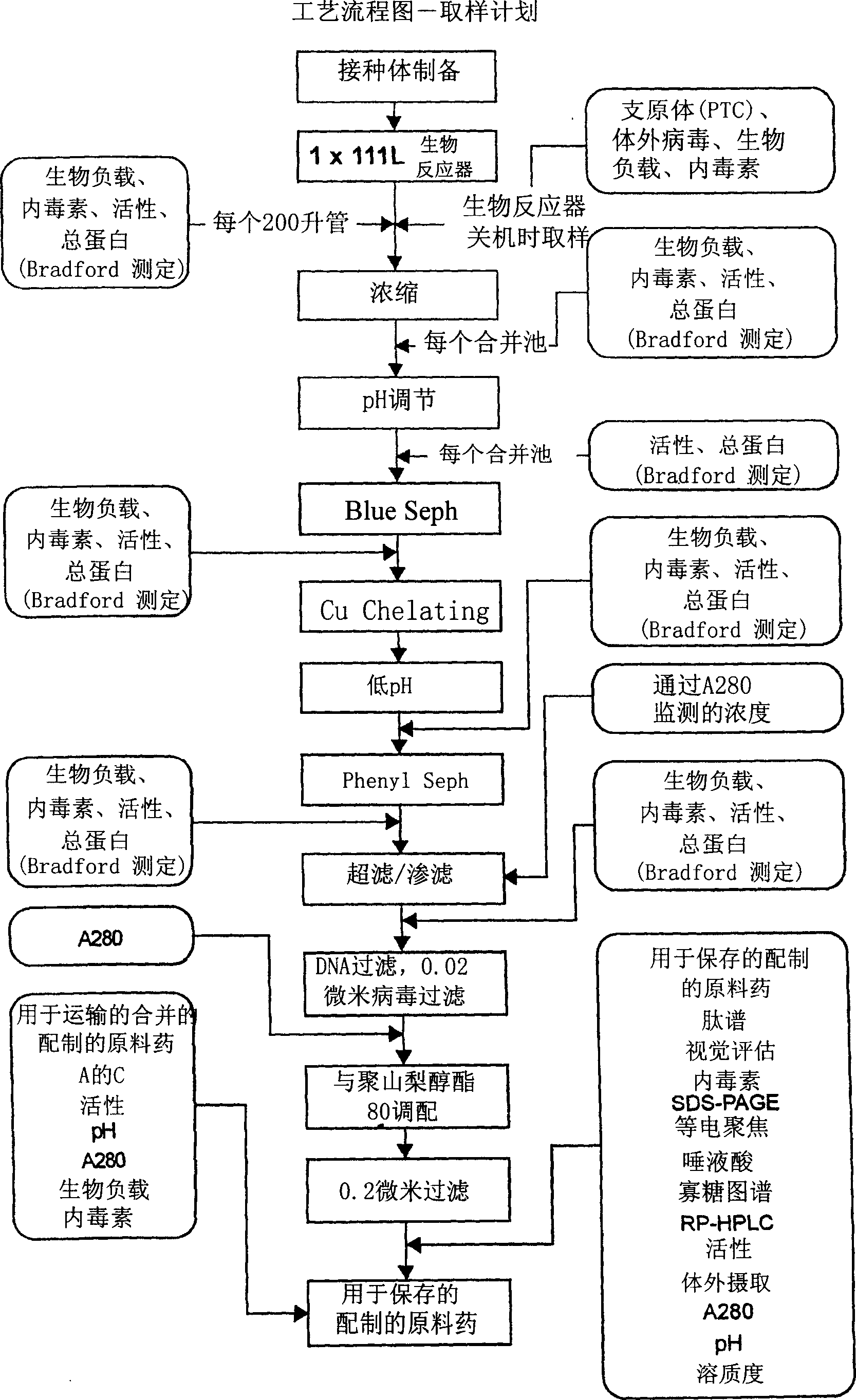
Precursor N-acetylgalactosamine-4 sulfatase, methods of treatment using said enzyme and methods for producing and purifying said enzyme
A technology of acetylgalactosamine and sulfatase, applied in the field of biochemistry and molecular biology, clinical medicine, can solve the problem of not providing clinical dosage or drug formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Clinical evaluation of recombinant human N-acetylgalactosamine-4-sulfatase
[0109] overview
[0110] Recombinant human N-acetylgalactosamine-4-sulfatase (rhASB) is targeted for the treatment of MPS VI (also known as Maroteaux-Lamy syndrome). We plan a rhASB clinical development program that includes an initial open-label clinical trial that provides safety, pharmacokinetics, and response to weekly infusions of the enzyme in subjects and defined clinical endpoints evaluation of. This trial should be conducted for at least 3 months to collect sufficient safety information for the 5 evaluated patients. During this period, if the initial 1 mg / kg dose does not produce a reasonable reduction in excess urinary mucopolysaccharides or produces a significant immediate clinical effect, the dose should be doubled and maintained for an additional 3 months to determine safety and assess further efficacy .
[0111] Target
[0112] Our primary objective was to demonstrate the saf...
Embodiment 2
[0129] A comprehensive review of useful information on felines with MPS VI and related pharmacological and toxicological studies is presented below: Enzyme replacement therapy has become an effective treatment for several genetic disorders such as Gaucher disease, Fabry disease, and mucopolysaccharide storage disorders type I. Promising treatments for metabolic disorders. In some of these disorders, natural animal models provide the ability to predict the clinical efficacy of human treatments in preclinical testing. This is true in MPS I (canine model). With this in mind, felines with MPS VI were studied before initiating human studies of the disease. Adequate safety and efficacy data exist to support continued clinical trials in human MPS VI patients.
[0130]A study of rhASB in cats with MPS VI noted that no cats died as a result of the administration. As predicted, studies in cats with MPS VI demonstrated that rhASB uptake is dependent on the presence of mannose-6-phosph...
Embodiment 3
[0140] Distribution and Feasibility
[0141] Initial studies were performed to illustrate the uptake and distribution of the enzyme and as a lead study for possible endpoints of further efficacy studies (Crawley et al., J. Clin. In.vest. 91, 864-1873 (1996)). Recombinant human ASB was administered weekly or biweekly to disease-affected cats at a dose of 0.5-1.5 mg / kg by bolus injection. An untreated MPS VI cat (Cat D) was evaluated as well as a normal cat for which values for comparison are provided. Data from untreated cats were further supported by historical evaluation data from an additional 38 untreated cats. Sensitive enzyme uptake and distribution studies were performed in normal cats using an immunoassay that allows detection of human ASB in the presence of normal cat enzymes.
[0142] The main conclusion of these studies illustrates the broad uptake of this enzyme, with the liver and spleen having an expected significant advantage in uptake as observed in other en...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com