Inhibitor of tricycro beta lactamase and preparation method
A technology for lactamase and inhibitors, applied in the field of tricyclic β-lactamase inhibitors and its preparation, can solve the problems of curative effect reduction and failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
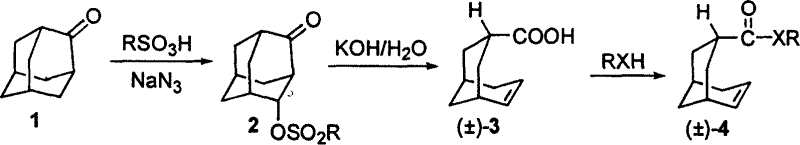
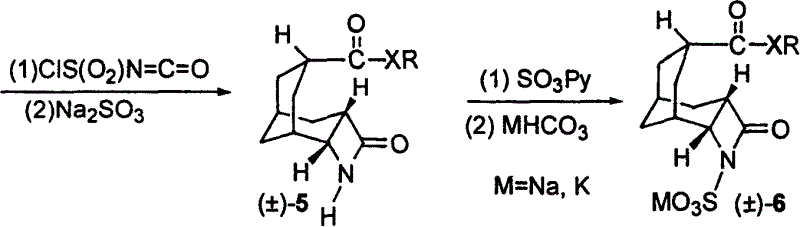
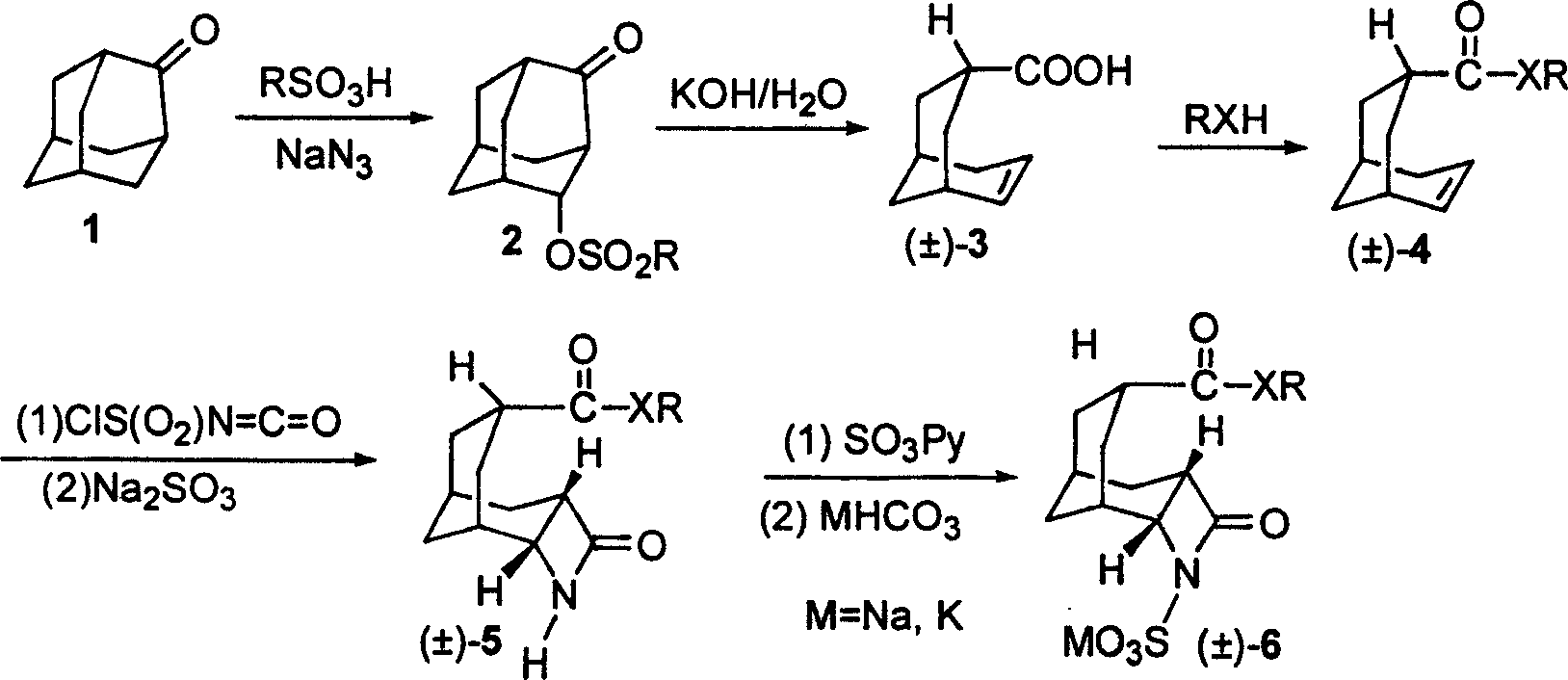
[0020] 1) Preparation of 4-sulfonyloxyadamantan-2-one (2)
[0021] Mix 2-adamantanone (1) and ethanesulfonic acid at a molar ratio of 1:1.0 evenly, add sodium azide equivalent to 1.4 molar times of (1) at room temperature, and continue to react at 35°C until the conversion of raw materials is complete , then the reaction mixture was poured into crushed ice, neutralized with saturated aqueous sodium bicarbonate, and extracted 3 times with chloroform, the combined extracts were washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and evaporated under reduced pressure to remove the solvent The resulting crude product was recrystallized from ethyl acetate / hexane to give 4-sulfonyloxyadamantan-2-one (2);
[0022] 2) Preparation of bicyclo[3.3.1]non-6-ene-3-carboxylic acid (±)-(3)
[0023] 4-sulfonyloxyadamantan-2-one (2) is dissolved in dichloromethane to make a 0.8 mol / liter solution, and 20% potassium hydroxide aqueous solution corresponding to (2) 4.5 mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com