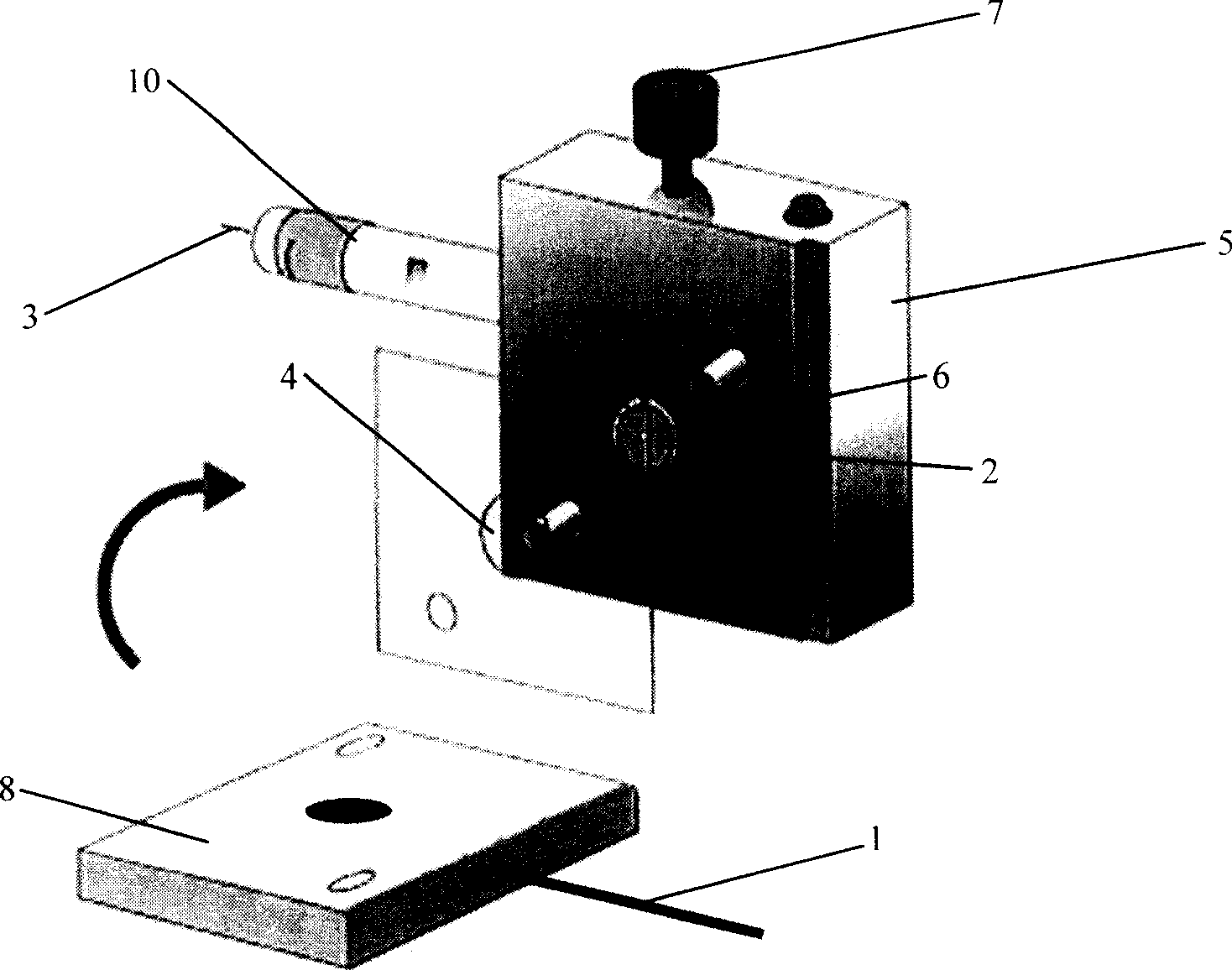
Method for realizing protein separation detection by using PVP/CdS quanta dot modified electrode
A technology for protein separation and electrode modification, which is applied in the field of electrodes, can solve the problems of limitation and slow electron transfer speed, and achieve the effects of low detection limit, high sensitivity and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 One of the methods for protein separation and detection using PVP / CdS quantum dot modified electrodes
[0035] The protein chromatographic column is VARIAN Microsorb 300-5C4 chromatographic column of American VARIAN Company.
[0036] In the second step, the flow rate of the mobile phase is 1.0mL / min, the mobile phase changes at a rate of 2% / min within the gradient range of 20% to 60% A, and the working voltage applied to the ECD by the electrochemical workstation is -0.3V , pre-run for 10 minutes. In the third step, configure the standard concentration as 1.0×10 -8 mol / L~1.0×10 -4 mol / L solution of three proteins: hemoglobin, myoglobin and cytochrome c, the peak times of the chromatographic peaks of the three proteins: hemoglobin, myoglobin and cytochrome c are 12.49min, 9.02min and 6.33min respectively , and its concentration-current working curves are respectively y=1.0841x+2×10 -8 , y=1.1537x+1×10 -8 and y=1.2451x+1×10 -8 , where y and x are the respo...
Embodiment 2
[0037] Example 2 The second method of using PVP / CdS quantum dot modified electrode to realize protein separation and detection
[0038] In the second step, except that the following conditions are different from Example 1, other conditions are the same as Example 1: the flow rate of the mobile phase is 2.0mL / min, and the mobile phase is in the range of 20%~60%A gradient at 4% / Min speed change, pre-run 12min. In the third step, the peak times of the chromatographic peaks of hemoglobin, myoglobin and cytochrome c are 6.25min, 4.51min and 3.17min respectively; Three chromatographic peaks appeared at 3 and 6.25 minutes respectively, and the peak currents were 0.73μA, 0.51μA and 0.89μA, respectively. By comparing with the standard peak time measured in the third step, it can be determined that the proteins to be tested corresponding to the three chromatographic peaks are cytochrome c, myoglobin and hemoglobin respectively. By comparing with the working curve measured in the thir...
Embodiment 3
[0039] Example 3 Method 3 of Using PVP / CdS Quantum Dot Modified Electrode to Realize Protein Separation and Detection
[0040] In the second step, except that the following conditions are different from Example 1, other conditions are the same as in Example 1: the flow rate of the mobile phase is 5.0mL / min, and the mobile phase is in the range of 20%~60%A gradient at 10% / min. Min speed change, pre-run 15min. In the third step, the peak times of hemoglobin, myoglobin and cytochrome c were 2.49min, 1.80min and 1.23min, respectively. In the fourth step, after the mixed sample 3 was injected, three chromatographic peaks appeared at 1.23min, 1.80min and 2.49min respectively, and the peak current values were 98nA, 75nA and 84nA, respectively. By comparing with the standard peak time measured in the third step, it is determined that the proteins to be tested corresponding to the three chromatographic peaks are cytochrome c, myoglobin and hemoglobin respectively. By comparing with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com