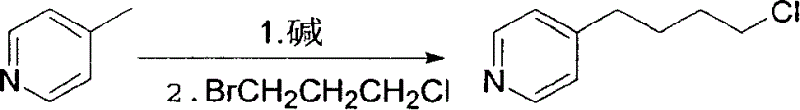
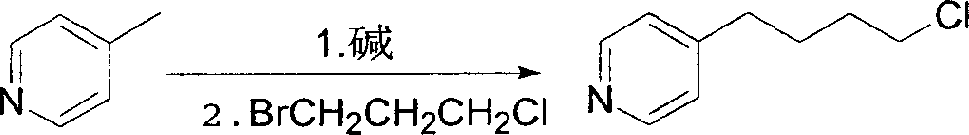Synthesis process of Tilofiban hydrochloride intermediate
A technology for tirofiban and intermediates, which is applied in the field of key intermediates for the preparation of tirofiban hydrochloride, which can solve the difficulties in industrial preparation, storage and transportation of butyllithium, and the difficulty in industrial production of tirofiban hydrochloride, hydrochloric acid Eliminate the high cost of tirofiban, and achieve the effects of good yield and quality, easy operation, and avoiding low-temperature reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0009] The preparation method of the present invention is further described in detail below by way of examples.
[0010] 1. Preparation of 4-(4-pyridyl)butyl chloride:
[0011] In a 5L three-neck bottle, pass dry nitrogen protection, add 1.6L of anhydrous ether, 1250ml of diisopropylamine, 50g of lithium metal, and add dropwise a solution of 460ml of styrene and 300ml of anhydrous ether in a water bath heated to reflux , after the addition, reflux and stir for 2 hours, the lithium flakes completely disappeared, and a gray solution was obtained.
[0012] The reaction solution was cooled with ice water to below 5°C, 465 g of 4-picoline (5 mol, diluted with 300 ml of anhydrous ether) was added dropwise, and the reaction was stirred at room temperature for 2 hours. Cool the reaction solution with ice water to below 5°C, add 820 g (5.2 moles) of 3-bromo-1-chloropropane dropwise, rise to room temperature and stir the reaction, monitor by TLC until the reaction is complete, add 400 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com