A topical nanoparticulate spironolactone formulation
A nanoparticle and spironolactone technology is applied in aerosol delivery, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. It can solve the problem of narrow particle size range, difficult preparation technology, and product stability. counterproductive issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of nanoparticulate spironolactone as a topical formulation
[0061] Preparation of nano-particle spironolactone
[0062] Table 1 shows a typical preparation method of nanoparticle spironolactone incorporated into a crystal structure according to the present invention. Nanoparticle spironolactone can be prepared as follows:
[0063] Under magnetic stirring, combine the aqueous formulation of the stabilizer with water or injection buffer until a clear solution is obtained; wet the spironolactone with an aqueous solution of a suitable amount of surfactant to form a slurry; the resulting suspension is dispersed by high shear The instrument is used for dispersion; the suspension is magnetically stirred to eliminate bubbles; the obtained suspension is passed through a high-pressure piston gap homogenizer to obtain nano suspensions. Use Avestin C5 TM Prepare preparations 1-7, using Avestin C50 TM Preparation 8-9 was prepared. In the homogenization proces...
Embodiment 2
[0123] Example 2: The size of spironolactone particles before and after storage
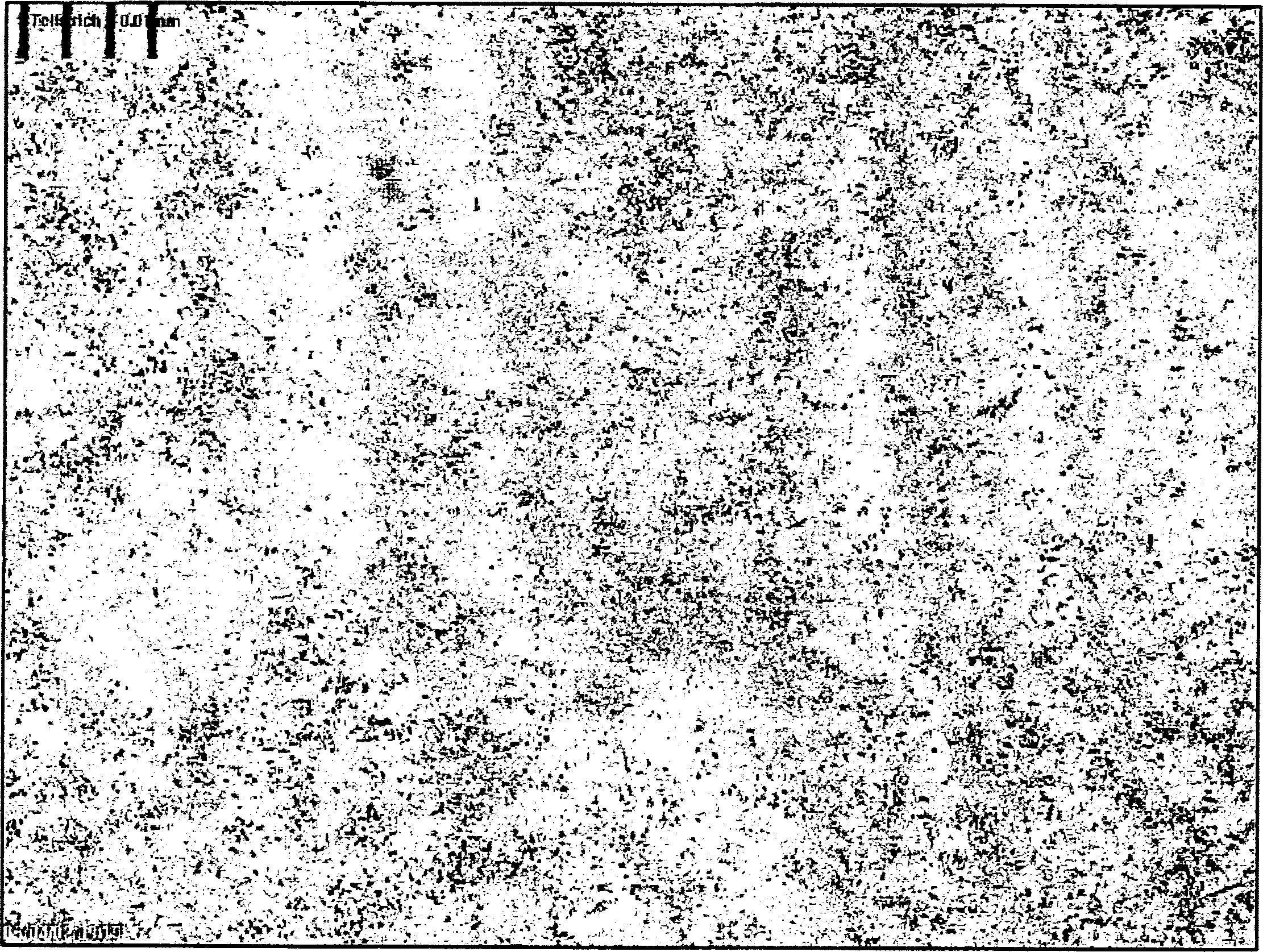
[0124] figure 1 , 2 And 3 are the microscope images of the spironolactone of the present invention just after preparation, after 7 months of storage, and compared with commercially available spironolactone. The distance between each vertical grid in the figure is 0.01 mm or 10 microns.
[0125] figure 1 with 2 The particles shown in are too small to be barely visible under an optical microscope. The pellets no longer grow up after 7 months of storage. In contrast, the commercially available spironolactone "Spiroderm" ( image 3 ) There are 20 micron spironolactone crystals.
Embodiment 3
[0126] Example 3: Liquidity research
[0127] In the Franz diffusion cell, the measurement results from the "Crystalip TM The flux of the spironolactone (2%) formulation of the matrix-bound nanosuspension formulation through the artificial membrane was compared with the 2% by weight spironolactone in the aqueous cream BP (Aqueouscream BP) as a control .
[0128] Raw materials
Provider
Crystalip TM Spironolactone nano-suspensions 2% by weight
The batch number is 4014-000 / 06atc
Lot number is 510 / 0
SkyePharma, Switzerland
Aqueous cream B.P.
Lot number is 28076
Hillcross, UK
R grade analytical pure
VWR International Ltd., UK
Sodium Dihydrogen Phosphate Dihydrate
Lot number is L298
Merckeurolab
Deionized water
Elga Ltd., UK
Acetonitrile HPLC grade
Rathburns Chemicals Ltd., UK
Regenerated cellulose membrane
NBS-Biological, UK
[0129] Metho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
| Crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com