Process for the production of 2'-branched nucleosides
A technology of nucleosides and ester nucleosides, applied in the field of preparation of 2′-branched nucleosides, achieving simple purification steps, high yields, and short cycle times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0157] Preparation of ribonolactones
[0158]
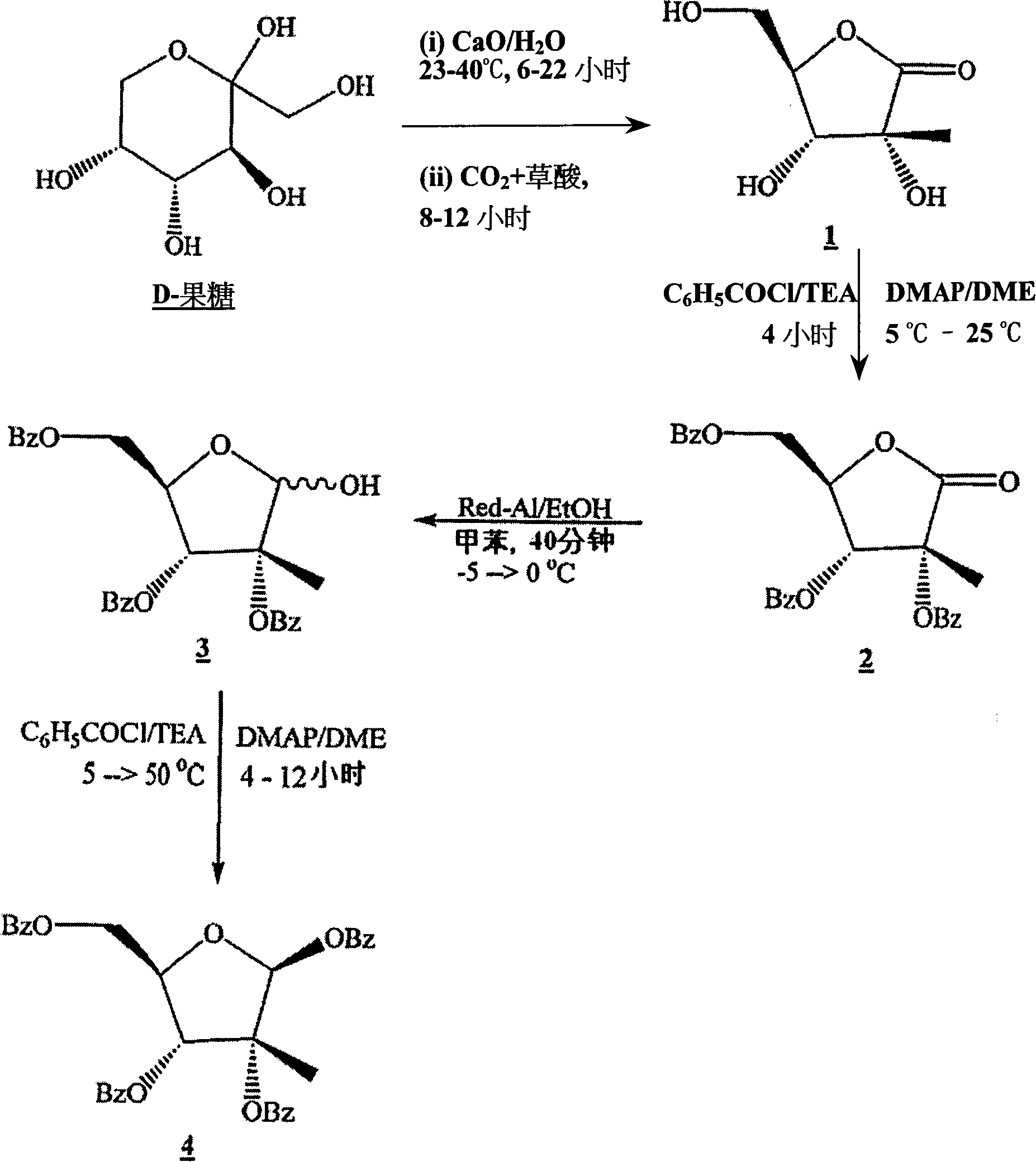
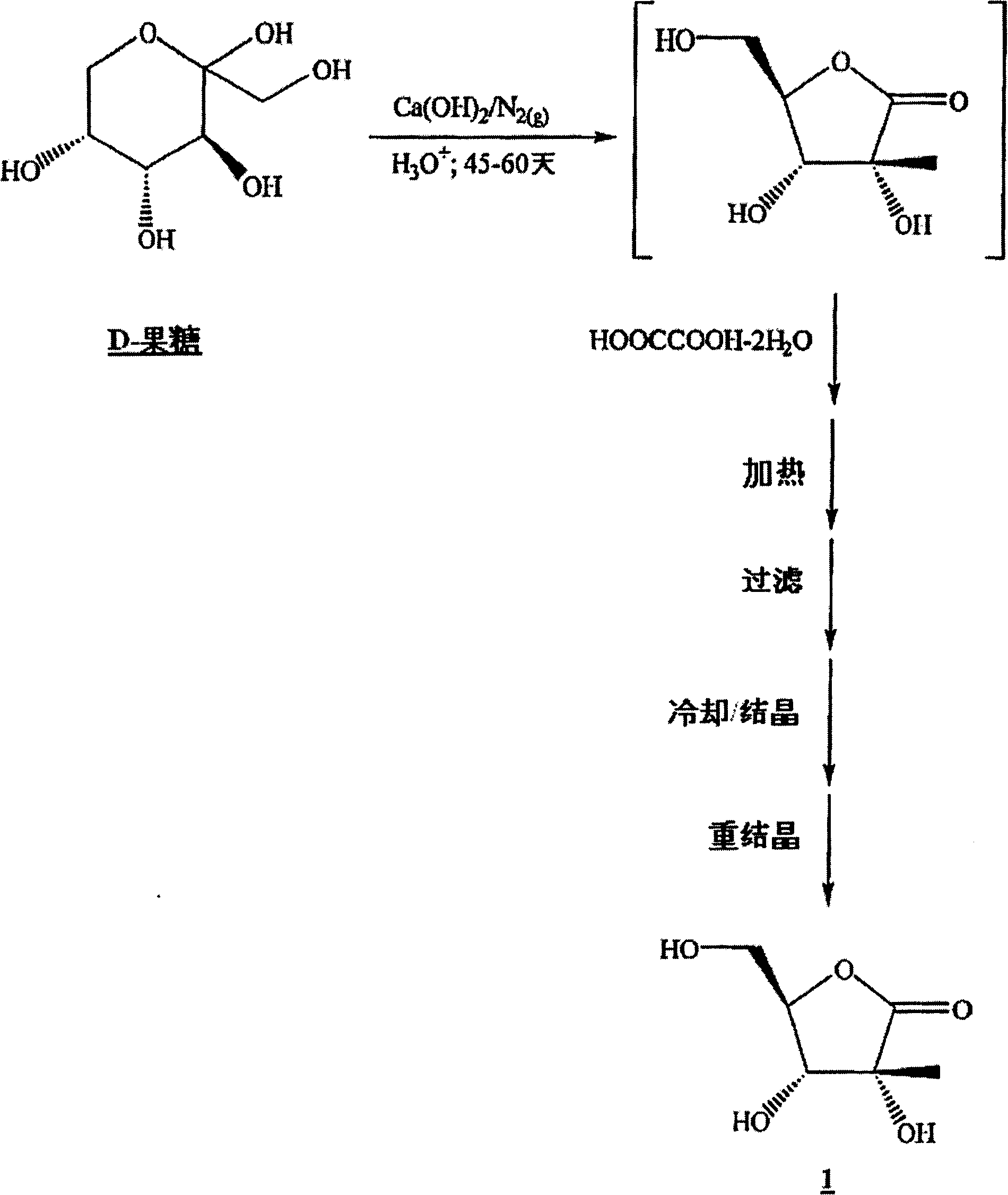
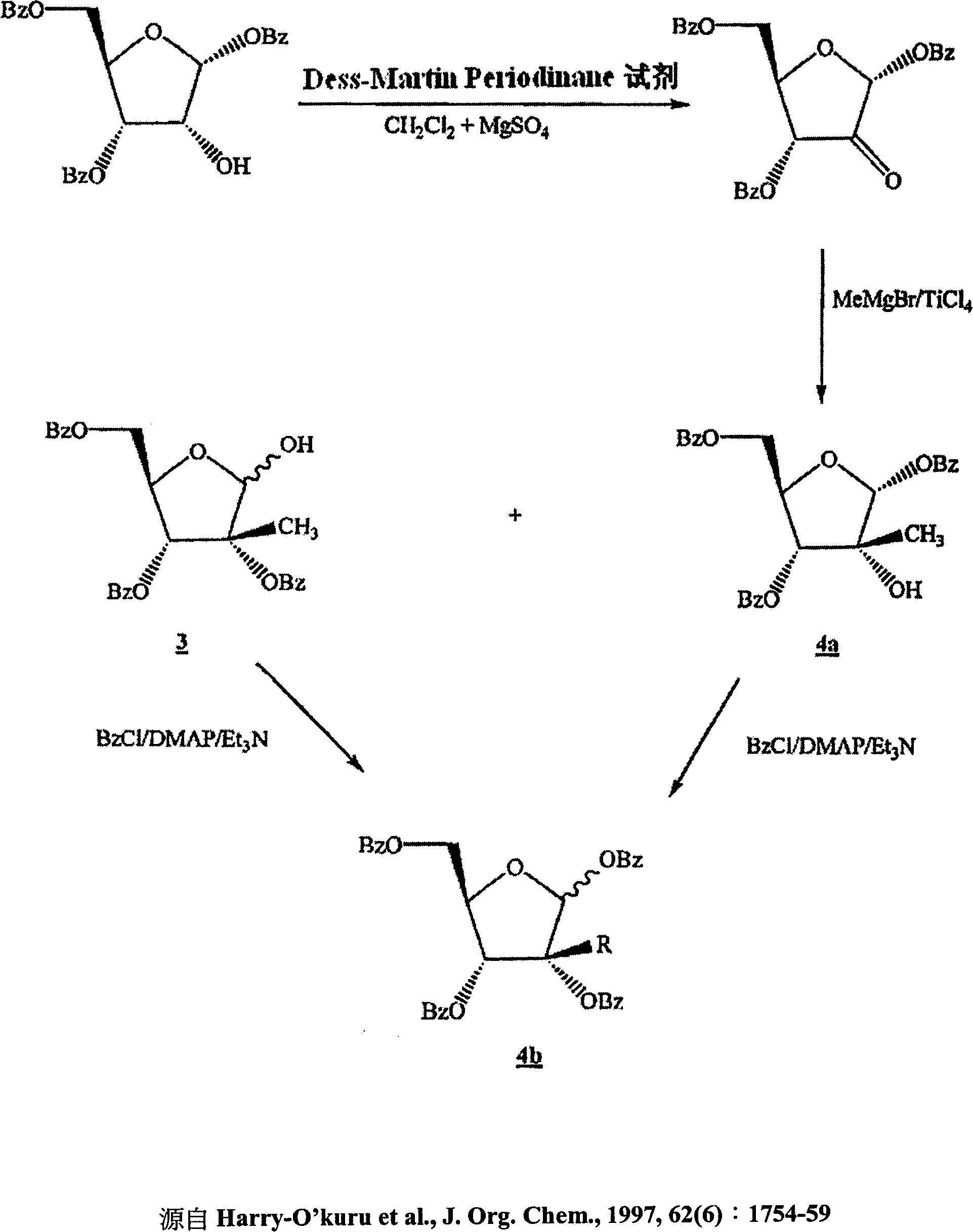
[0159] Ribonolactones can be prepared by any published or unpublished method, including standard oxidation and substitution techniques. One embodiment of the ribonolactone synthesis of the present invention is through D-fructose, according to the following steps.
[0160] Ribonolactone can be prepared by the reaction of D-fructose with calcium oxide (CaO). D-fructose can react with CaO in any molar ratio provided that the reaction proceeds at an acceptable rate without excessive by-products. For D-fructose, the preferred molar ratio is 5:1, and the more preferred molar ratio is 3:1, the most preferred molar ratio is 2.3:1.3. The CaO can be added at any rate provided that the reaction proceeds at an acceptable rate without generating excess heat or excessive by-products. In one embodiment, CaO is added incrementally over 5 minutes at room temperature. The reaction can be carried out until the D-fructose is almost consumed, ...
Embodiment approach
[0199] One embodiment of the invention includes a method of synthesizing the 3'-esters of β-D-2'-C-methyl-cytidine, in particular the following 3'-esters of β-D-2'-C-methyl-cytidine '-valine ester method.
[0200] The 3'-ester of β-D-2'-C-methyl-cytidine can be prepared by optionally protecting the amino group of β-D-2'-C-methyl-cytidine by any known method. For example in Greene, et al., Protective Groups in Organic Synthesis , the method taught in John Wiley and Sons, Second Edition, 1991. In one embodiment of the invention, β-D-2'-C-methyl-cytidine can be combined with Me 2 NCH(OMe) 2 Reaction in DMF yields N[1-(3,4-dihydroxy-5-hydroxymethyl-3-methyl-tetrahydrofuran-2-yl)-2-oxo-1,2-dihydro-pyrimidine-4 -yl]-N,N-dimethylformamidine.
[0201] In a specific embodiment, the compound can be further protected with TBDPSCl and imidazole to provide the 5'-silyl-protected compound, N'-{1-[5-(tert-butyl-diphenyl-silyloxy methyl)-3,4-dihydroxy-3-methyl-tetrahydrofuran-2-yl]-2...
Embodiment 1
[0223] 2-C-Methyl-D-ribo-γ-lactone
[0224] In a 250 mL 3-neck round bottom flask equipped with an overhead stirrer, stirrer shaft, digital temperature readout, and argon passage, stir deionized water (100 mL). Argon was bubbled through for 30 minutes, D-fructose (20.0 g, 0.111 mol) was added and the solution became clear within a few minutes. Calcium oxide (12.5 g, 0.223 mol) was added in portions over 5 minutes and the mixture was stirred vigorously. An exotherm was observed and the reaction temperature rose to 39.6°C after 10 minutes from the addition of calcium oxide. After about 15 minutes, the reaction mixture turned yellow and darkened with time. After 3 hours, a portion of the reaction mixture was removed for TLC analysis. The removed fraction was acidified to pH 2 with saturated aqueous oxalic acid. The resulting white suspension was distilled off of water under reduced pressure. Toluene (2 mL) was added to the residue, and the mixture was distilled under reduced...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com