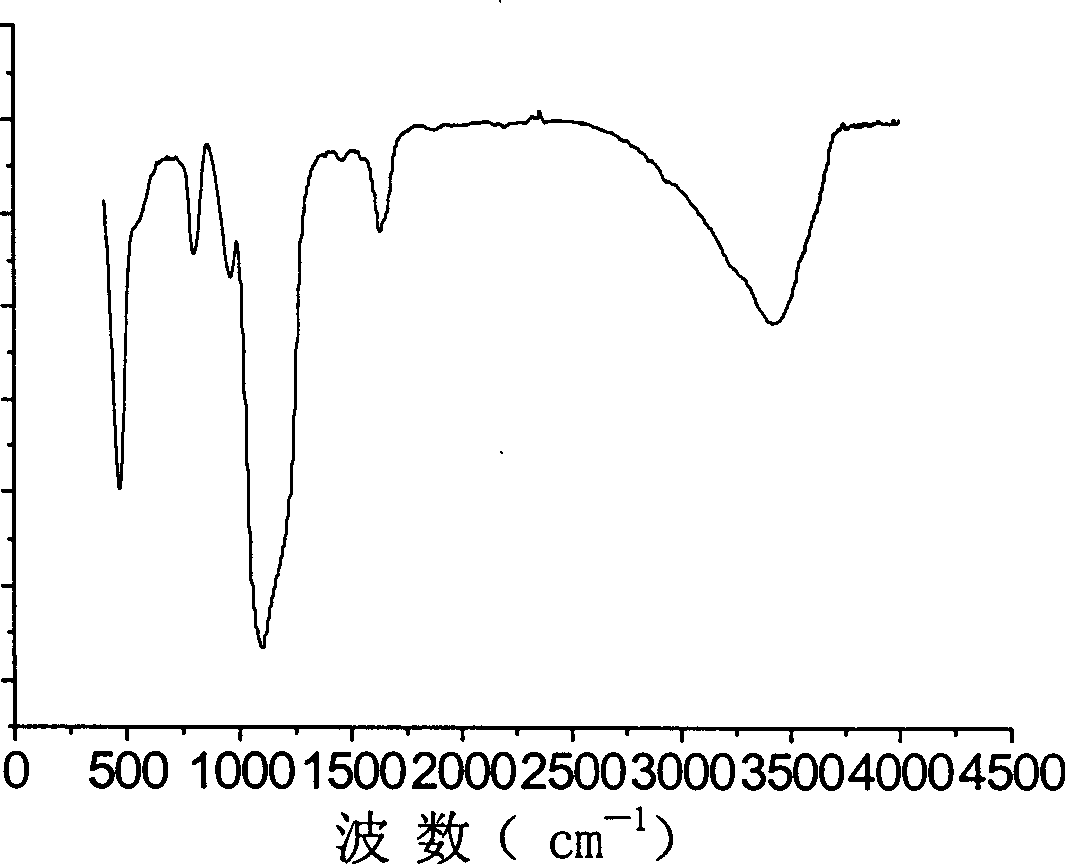
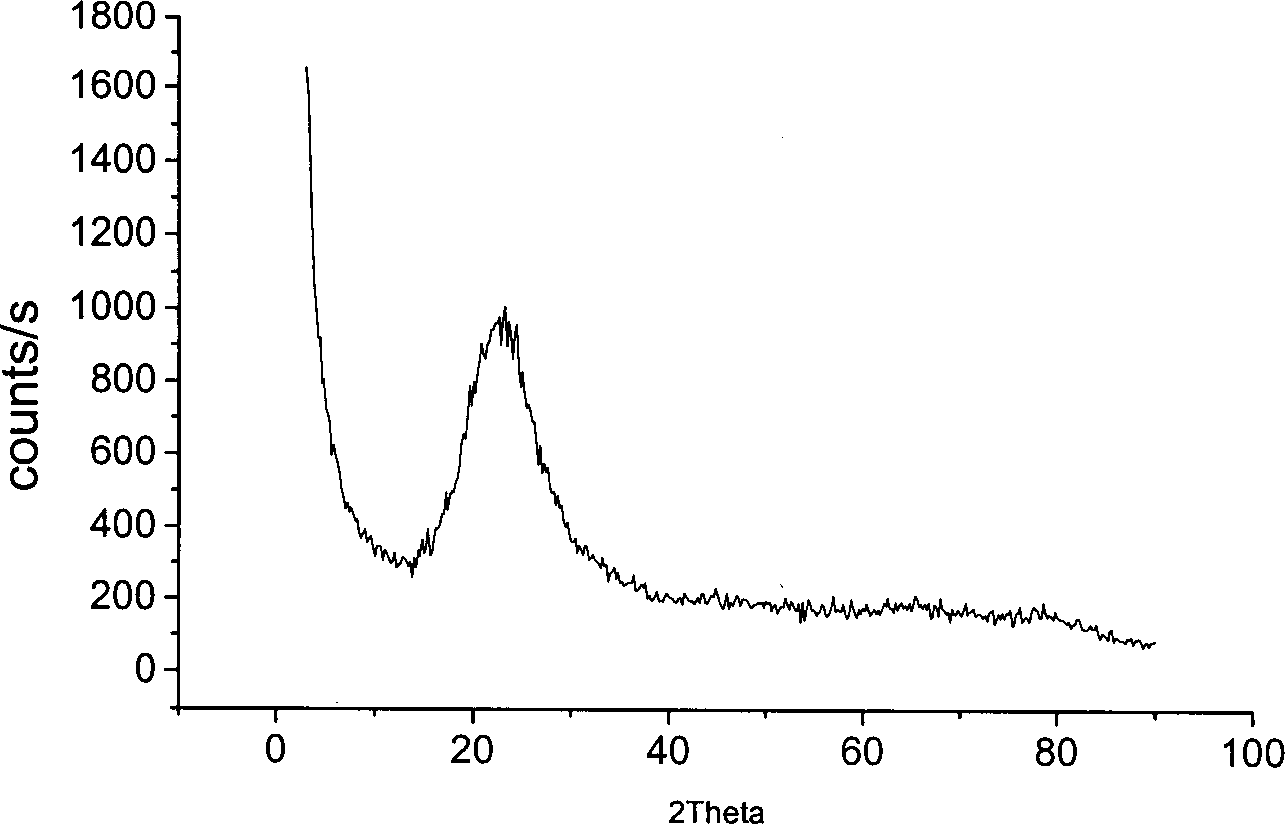
Method for preparing superfine silicon dioxide powder using sulfuric acid precipitation reaction
A technology of ultra-fine silica and precipitation reaction, applied in the direction of silica, silica, etc., to achieve the effect of high industrial production value, shortened time and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
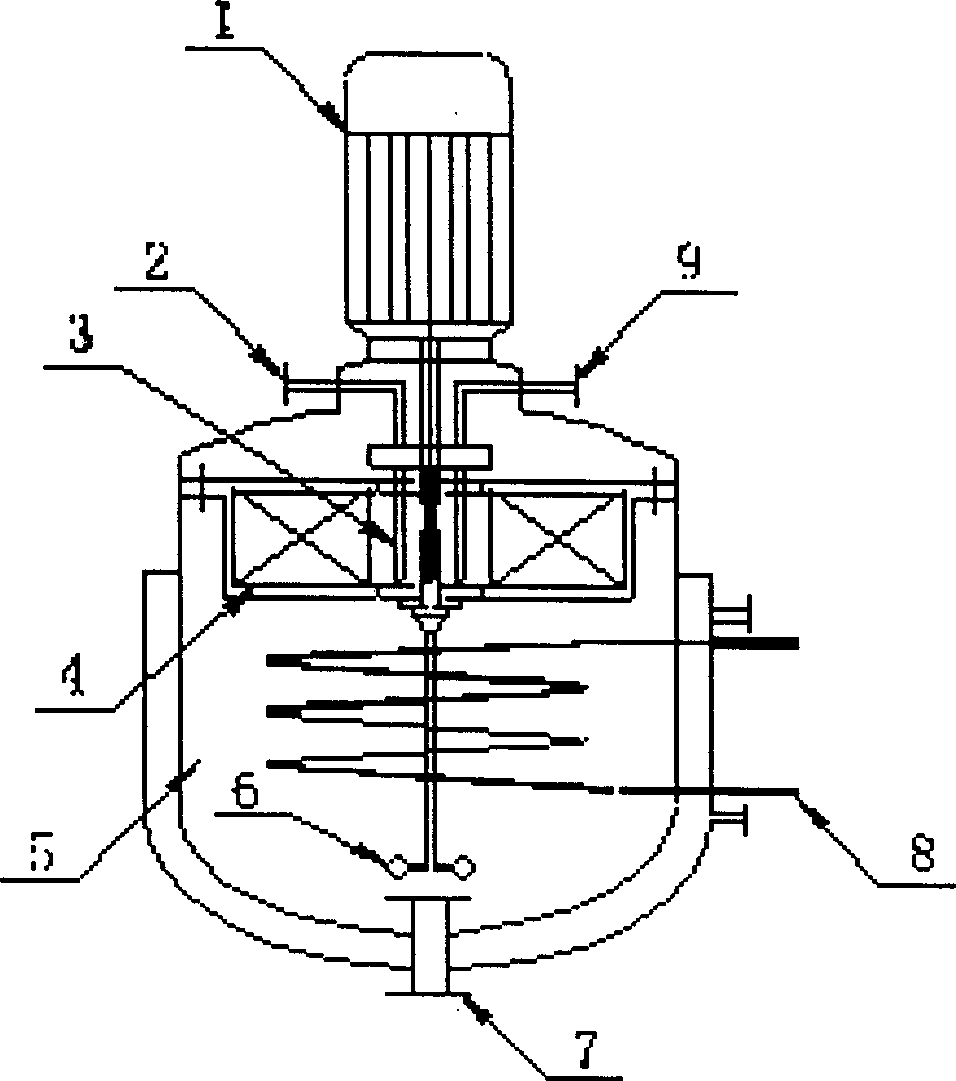
[0032] Such as figure 1 As shown: this embodiment adopts the rotating bed supergravity field device (Chinese patent 00132275.3) developed by the applicant, the rotating packing layer 4 is driven by the motor 1, the rotating speed is regulated by the frequency regulator, and sulfuric acid is continuously added during the cycle to form a continuous cycle reaction. According to water glass: water=1: 7 (volume ratio) ratio preparation specific gravity is 10 liters of sodium silicate solution of 6.6 ° Be ', adds 150gNaCl after filtering to remove impurity, and is stirred to make it dissolve completely. Add the prepared feed liquid into the liquid holding tank 5 with the stirring paddle 6, heat the heating tube 8 and keep stirring, after the temperature rises to 90°C and stabilizes, start the feed liquid circulation pump, adjust the rotating bed speed to 900rpm, The material-liquid circulation pump enters the liquid circulation pipeline, and enters the inner cavity of the rotating...
Embodiment 2
[0034] Except for the following changes, all the other conditions were the same as in Example 1.
[0035] The rotation speed of the rotating bed is 1200rpm, the acid addition time is controlled within 3 minutes, the reaction time is 8 minutes, the average particle size of the primary particles of the product is 8 nanometers, and the specific surface area BET=307m 2 / g, bulk density is 0.12g / cm 3 .
Embodiment 3
[0037] Except for the following changes, all the other conditions were the same as in Example 1.
[0038] The concentration of sulfuric acid is 40%, the volume flow ratio of feed liquid and sulfuric acid is 200:1, and the acid addition time is controlled within 3 minutes, and the reaction time is 10 minutes. The average particle diameter of the primary particles of the product is 14 nanometers, and the specific surface area BET=210m 2 / g, bulk density is 0.178g / cm 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com