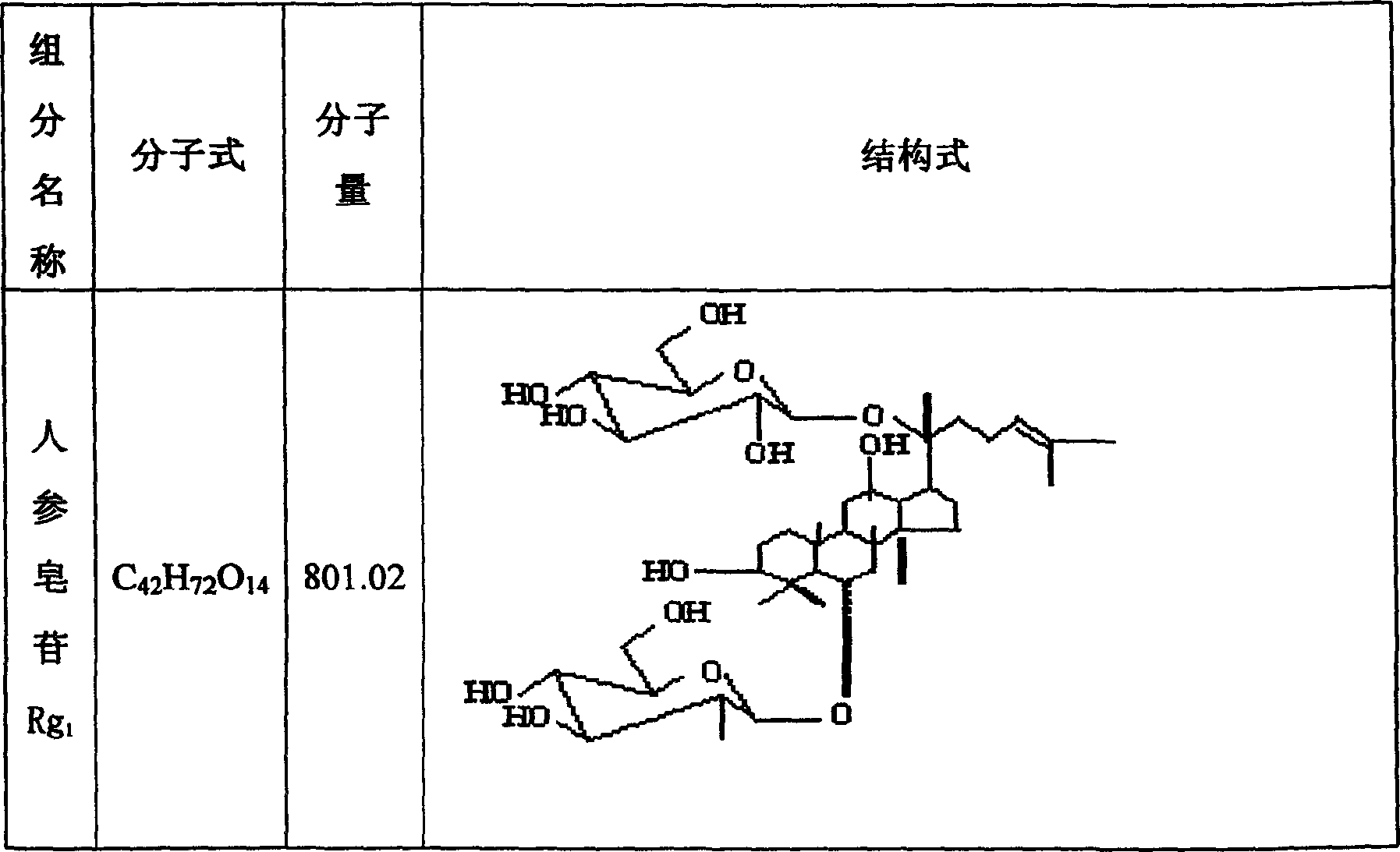
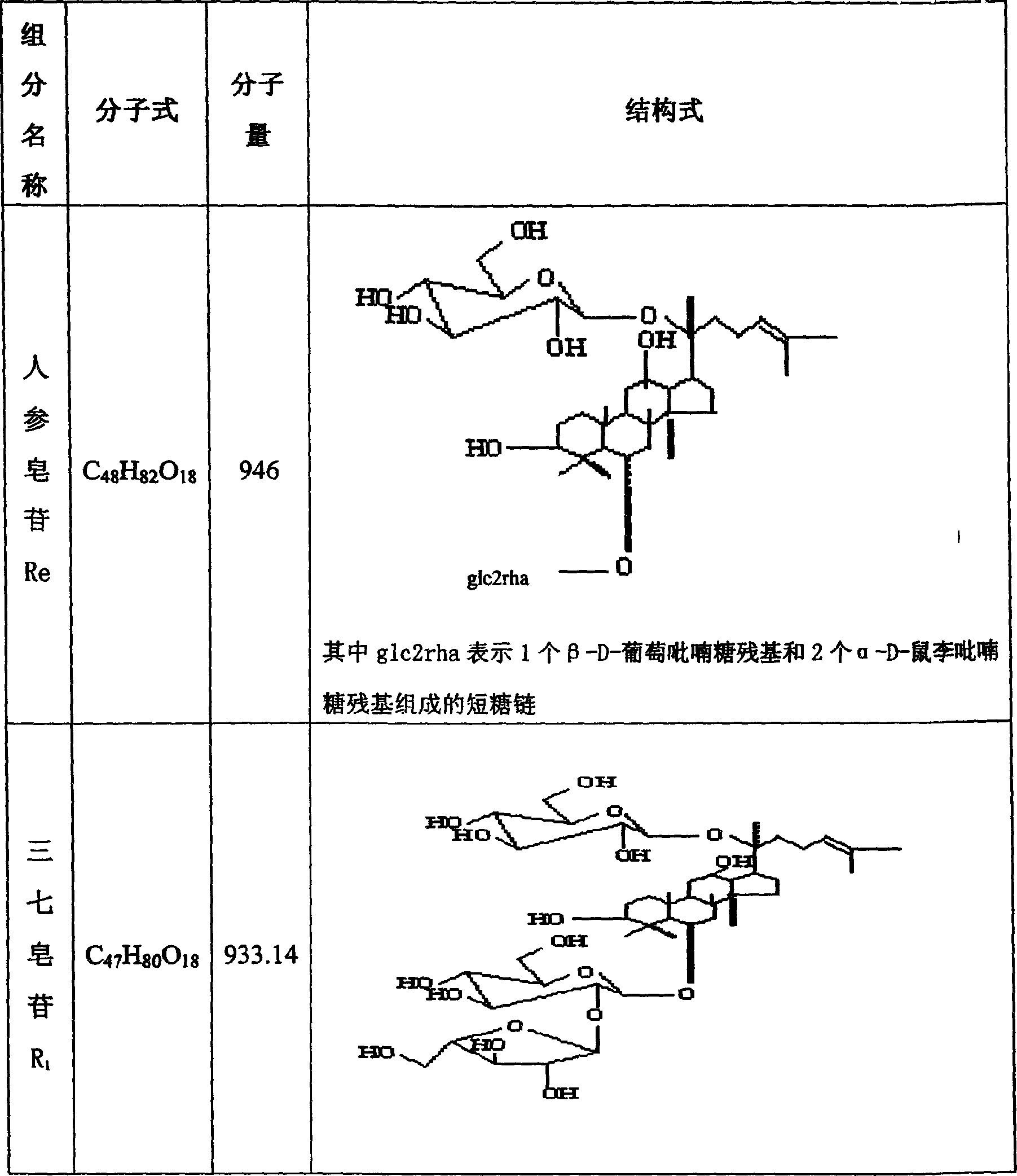
Notoginseng triol-saponin composition and its prepn and use
A technology of triol saponins and Panax notoginseng saponins is applied in the field of the purification of traditional Chinese medicine Panax notoginseng and its preparation, and achieves the effects of solvent safety, reducing whole blood viscosity and preventing thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Use a universal pulverizer (purchased from Shanghai Tianhe Pharmaceutical Machinery Factory, model GF-300) to pulverize 150 kg of dry rhizomes of Sanqi (place of origin: Wenshan, Yunnan Province), to a particle size range of 10 mesh to 24 mesh Sanqi coarse powder, use 2 The concentration of twice the weight of the medicinal material is 60wt% ethanol soaked in the above-mentioned Panax notoginseng coarse powder and then filled into the percolation barrel, and the concentration of 15 times the weight of the medicinal material is 60wt% 7 alcohol to percolate the wet material, and the ethanol in the percolation liquid is recovered under reduced pressure Afterwards, Panax notoginseng total saponin alcoholic extract. The above-mentioned Panax notoginseng saponin alcohol extract was prepared into a solution of 10 times the weight of the medicinal material with purified water, and the resulting aqueous solution was loaded onto the column of styrene-type macroporous resin D140 (p...
Embodiment 2~4
[0046] According to the same method and steps as described in Example 1, ethanol with different concentrations and different weights was used to soak, percolate and elute the Panax notoginseng coarse powder to obtain the following composition of the present invention, and the results are shown in Table 2 below Show:
[0047] Example
Embodiment 5
[0049] Get the dry PTS 100g that embodiment 1 makes, use the acetic acid buffer solution that pH value is 6 to be mixed with 2.5L solution, the gained solution is loaded on weakly basic anion exchange resin BS-II (purchased from Chengdu Zhonglan Chenguang Chemical Industry Co., Ltd. Research Institute) column, the volume of the resin column is 6L, eluted with 12L of acetic acid buffer solution with a pH value of 6, the flow rate is 0.4 times the column volume / hour, the eluate is collected, and the obtained eluate is concentrated and dried to obtain 60g of powder A refined notoginseng triol saponin composition.
[0050] By high performance liquid chromatography, octadecylsilane bonded silica gel is used as a filler, acetonitrile-water (volume ratio is 19.5:80.5) is used as a mobile phase, and the detection wavelength is 210nm, and the PTS obtained in Example 5 is measured respectively. Middle Rg 1 content of 65wt%, Re content of 8wt%, R 1 The content is 20wt%. The moisture c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com