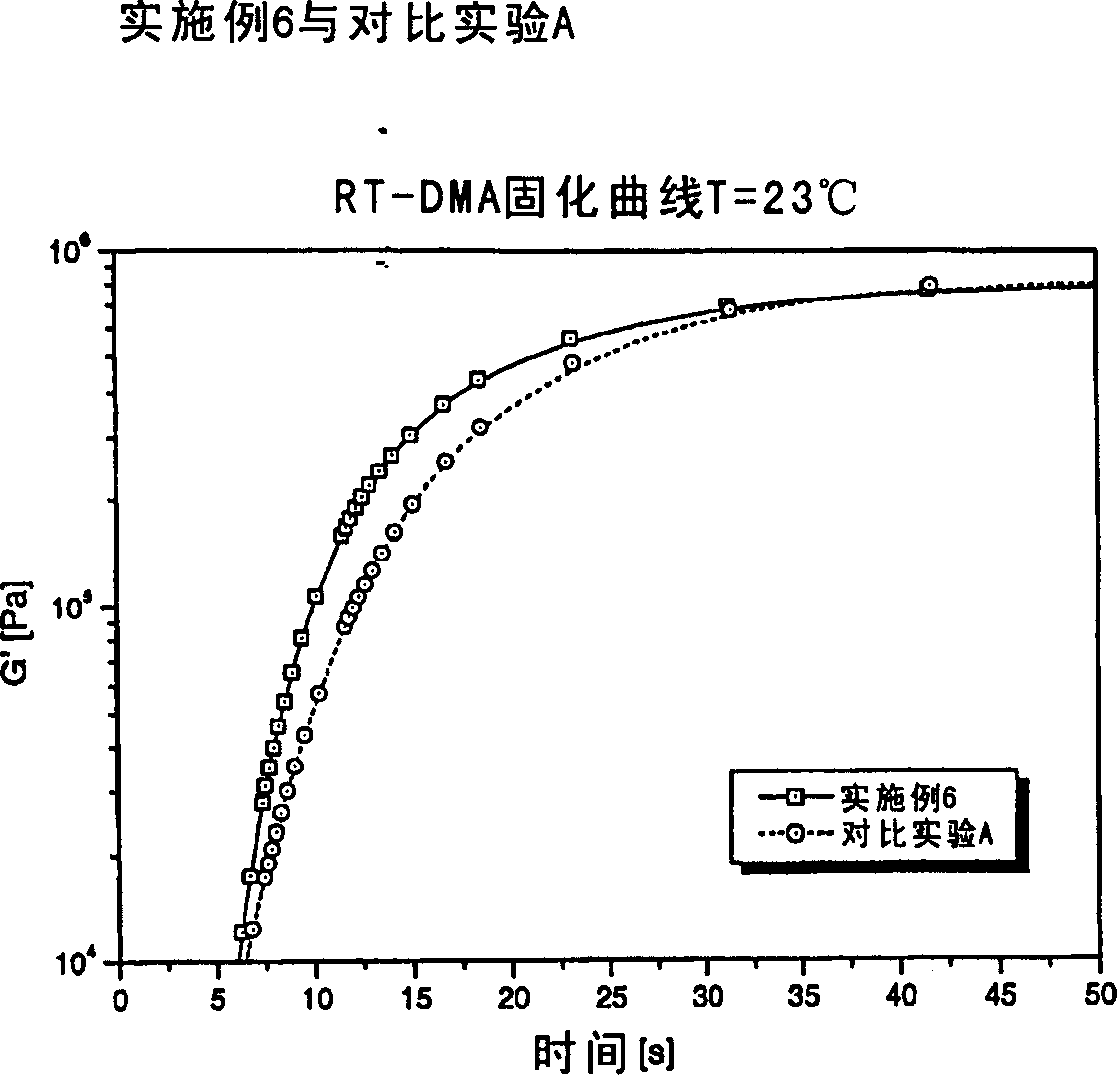
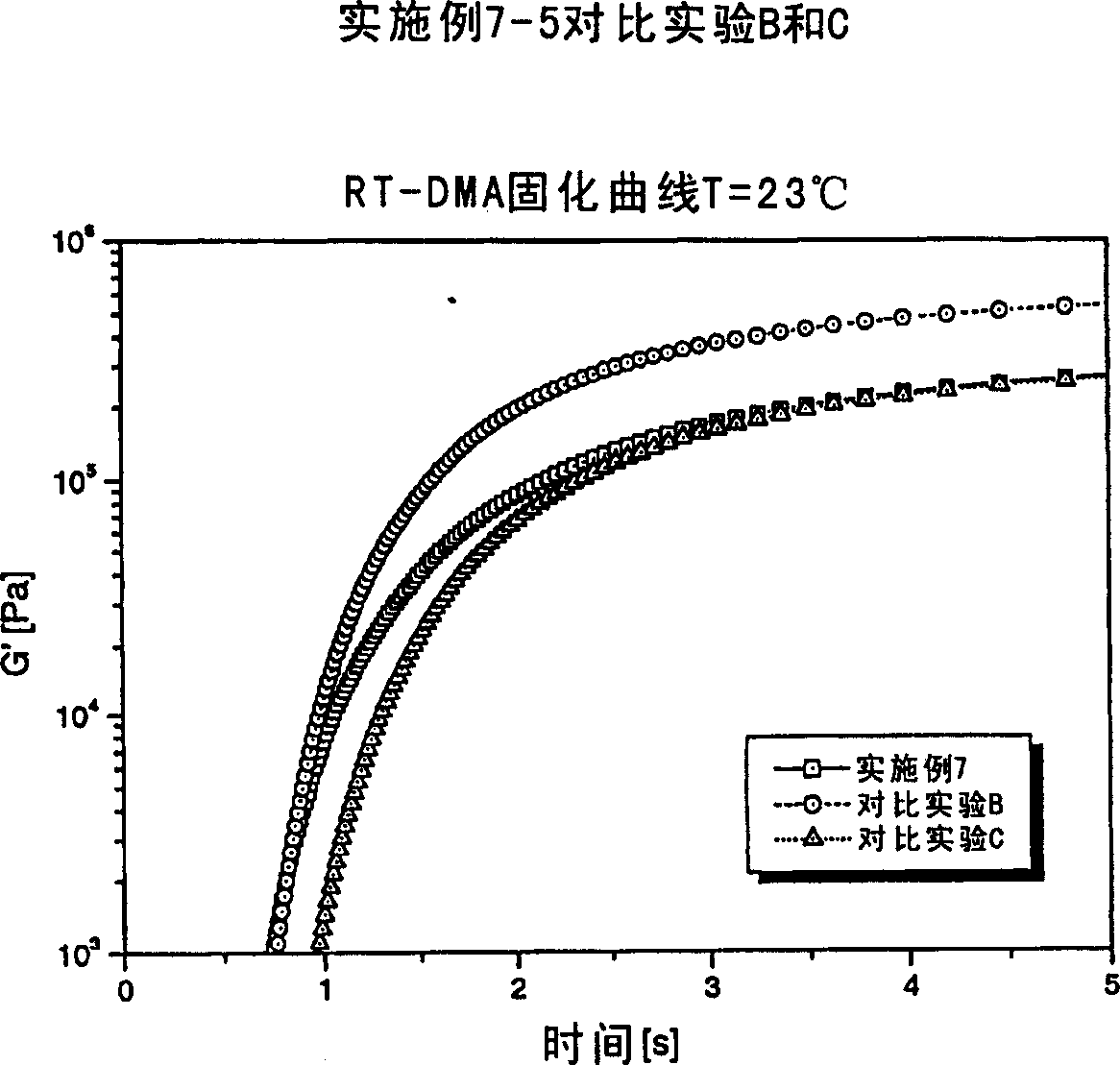
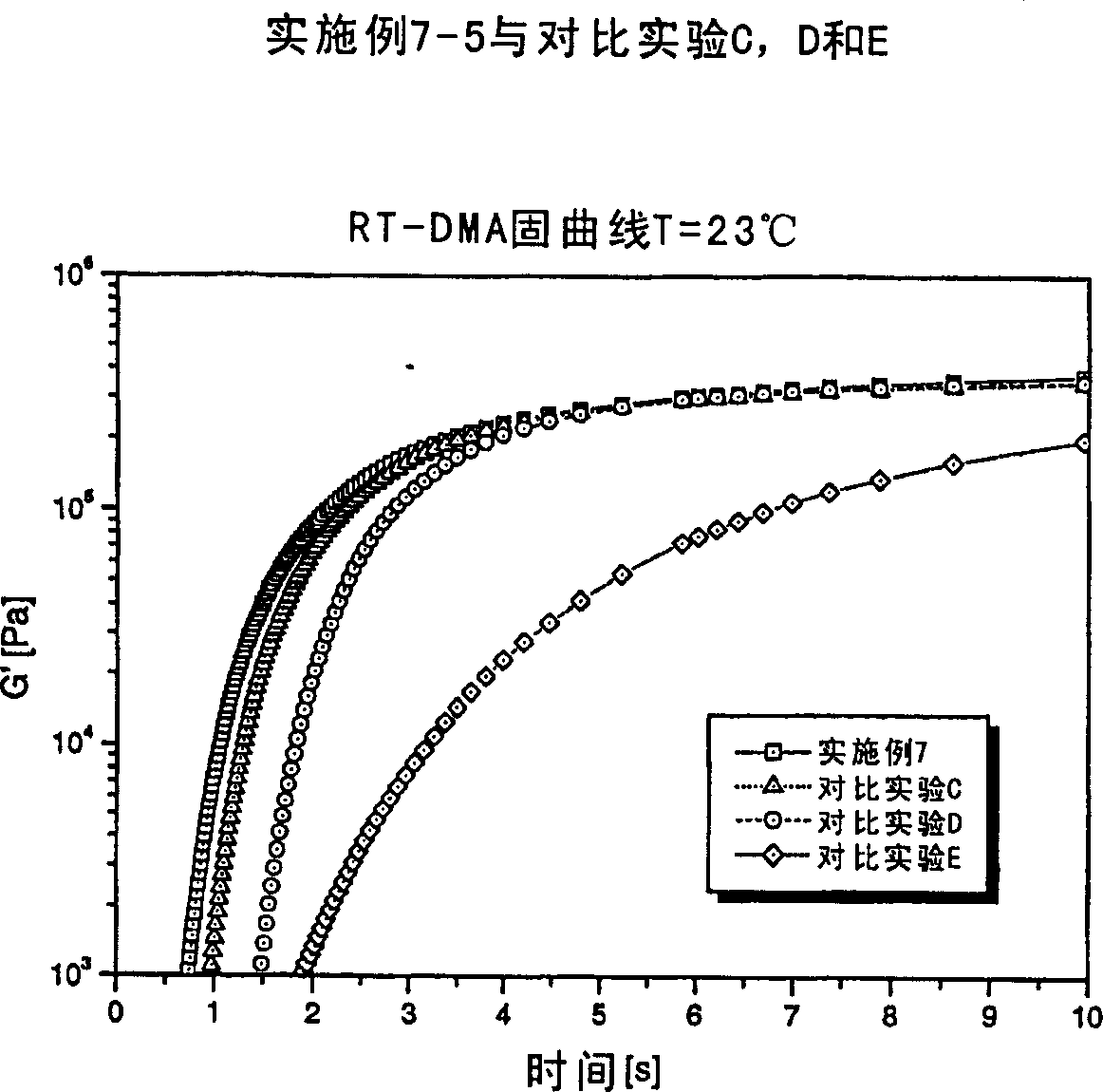
Radiation curable composition
A compound and composition technology, applied in the field of curable thiol-ene composition, can solve the problems of no curing speed, unsuitable for industrial production, complicated preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Example 1 Synthesis of PTGL 2000 carbamate norbornene oligomer
[0129] Dissolve 21.4 g (0.008 mol) of acrylate oligomer PTGL 2000 urethane diacrylate (made from PTGL 2000 diol, IPDI and HEA) in 15 mL of toluene, and in a nitrogen atmosphere at 40 ° C, in Stir in a 250mL three-necked round-bottom flask with a high-efficiency condenser, constant-pressure addition funnel, and thermometer. 1.56 g of freshly cleaved cyclopentadiene monomer (0.024 mol, 3 equiv) was added dropwise via an addition funnel. The reaction temperature was then raised to 80°C and stirred for about 16 hours (overnight). Finally, excess cyclopentadiene and dicyclopentadiene were removed under vacuum (0.5-2 mbar, below 70 °C) to obtain a light yellow resin in quantitative yield. After the reaction, FT-IR and 1 H NMR measurement.
[0130] FT-IR (pure, cm -1 ): 1722 (carbamate carbonyl); 3061.1, 712 (norbornene double bond)
[0131] 1 H NMR (CDCl 3 , ppm): 5.93, 6.12, 6.18 (outer hydrogen and inn...
Embodiment 2
[0135] Synthesis of embodiment 2 PPG 2000 carbamate norbornene oligomer
[0136] 21.4 g (0.008 mol) of acrylate oligomer PPG 2000 urethane diacrylate (prepared from PPG 2000 diol, IPDI and HEA as described in US-A-5837750) was dissolved in 10 mL of toluene, And stirred in a 250 mL three-necked round bottom flask with a high-efficiency condenser, a constant pressure addition funnel and a thermometer under a nitrogen atmosphere at 40°C. 1.56 g (0.024 mol, 3 equiv) of freshly cleaved cyclopentadiene monomer was added dropwise via an addition funnel, then the reaction temperature was raised to 80° C. and stirred for about 16 hours (overnight). Finally, excess cyclopentadiene and dicyclopentadiene were removed under vacuum (0.5-2 mbar, below 70° C.), and a yellowish resin was produced quantitatively. FT-IR and1 Analysis of the reaction mixture by H NMR measurements indicated that the conversion was complete.
[0137] FT-IR (pure, cm -1 ): 1722 (carbamate carbonyl); 3061.1, 712 (...
Embodiment 3
[0142] The synthesis of embodiment 3 PPG 4200 carbamate norbornene oligomers
[0143] The oligomer of Example 3 was prepared in the same manner as in Example 2, except that PPG 4200 diol was substituted for PPG 2000 diol. The resulting compound can be represented by the same chemical structure given in Example 2, but where the backbone is PPG 4200, X is derived from IPDI and n is equal to 1. The theoretical number average molecular weight Mn of the compound is 5009, which can also be expressed as PPG 4200-(IPDI-HEA-NB) 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| modulus | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com