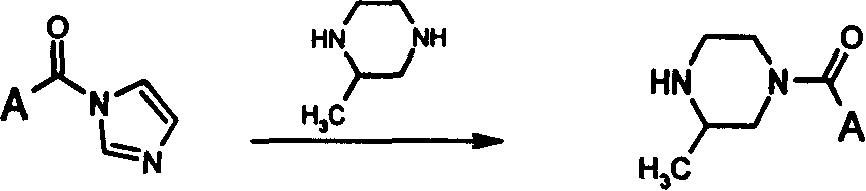
Process for preparing 4-acyl substituted-2-methylpiperazines
A methylpiperazine and acyl substitution technology is applied in the field of preparation of 4-position-acyl-substituted-2-methylpiperazine compounds, which can solve the problems that monoacylated products cannot be effectively separated and the like, and achieves the elimination of potential safety hazards. , the raw materials are cheap and easy to obtain, the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
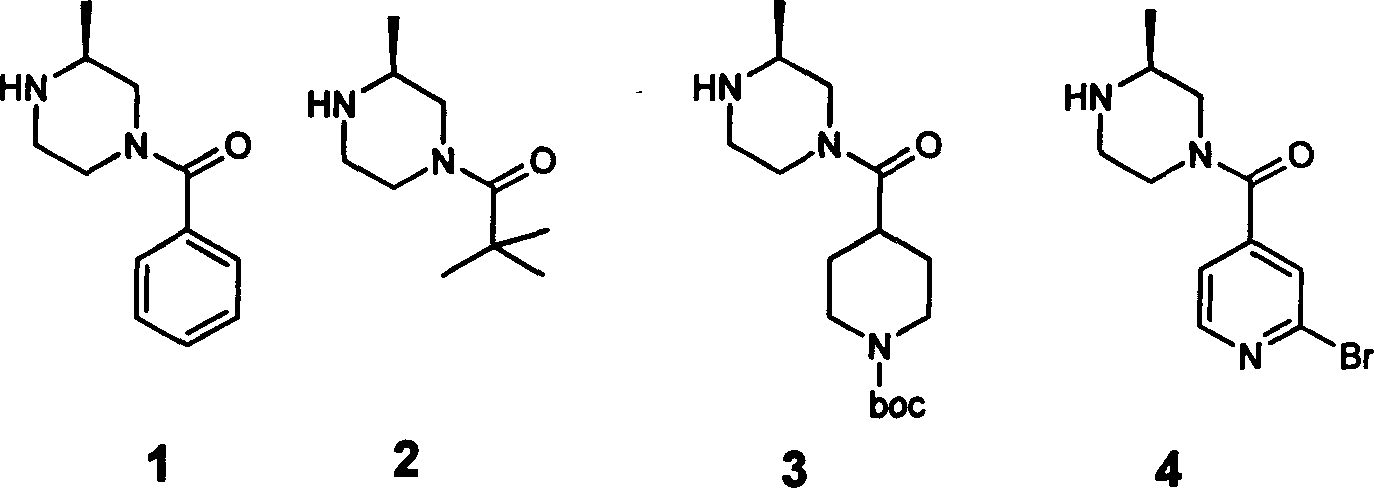
[0021] Synthesis of 4-benzoyl-2-methylpiperazine
[0022]
[0023] Benzoic acid (2.5g 0.02mol) and N,N'carbonyldiimidazole (3.3g 0.02mol) were dissolved in dry tetrahydrofuran 50mL and heated to reflux for 3 hours, and (R)-2-methylpiperazine (2.0g0. 02mol), after continuing to reflux for 3 hours, the reaction ended, and the reaction mixture was purified by column chromatography (eluent: ethyl acetate: methanol = 4: 1) to obtain the product 4-benzoyl-2-methylpiperazine 1.6 g, yield 40%. Low resolution mass spectrum (LCMS) M+1, 205.1. h 1 -NMR (300MHz, CD 3 OD): δ7.45 (m, 5H), 4.50 (d, 1H), 3.60 (b, 1H), 3.33-2.60 (m, 5H), 1.16-0.98 (d, 3H).
Embodiment 2
[0025] Synthesis of 4-pivaloyl-2-methylpiperazine
[0026]
[0027] Dissolve pivalic acid (0.02mol) and N,N'carbonyldiimidazole (0.02mol) in 50mL of dry tetrahydrofuran and heat to reflux for 3 hours. After adding (R)-2-methylpiperazine (0.02mol), continue After heating to reflux for 3 hours, the reaction was completed, and the reaction mixture was purified by column chromatography (eluent: ethyl acetate: methanol = 4: 1) to obtain the product 4-pivaloyl-2-methylpiperazine, with a yield of 1.5 g. Yield 40%. Low resolution mass spectrum (LCMS) M+1: 185.1. h 1 -NMR (300MHz, CD 3 OD): 4.32-4.24(m, 2H), 3.00-2.92(m, 2H), 2.76-2.65(m, 2H), 2.59-2.51(m, 1H), 1.26(s, 9H), 1.09(d, 3H).
Embodiment 3
[0029] Synthesis of 4-[4-(1-tert-butoxycarbonylpiperidine)formyl)]-2-methylpiperazine
[0030]
[0031] Add N-tert-butoxycarbonylpiperidine-4-carboxylic acid (3.84g, 0.0166mol) and N,N'carbonyldiimidazole (3.769g, 0.02324mol) into dry tetrahydrofuran (50mL), stir at room temperature until dissolved . At room temperature, (R)-2-methylpiperazine (1.829 g, 0.01826 mol) was added to the reaction solution, stirring was continued at room temperature for 24 hours, the reaction solution was concentrated under reduced pressure, and the concentrate was subjected to silica gel column chromatography (ethanol: Ethyl acetate=1:5) to obtain 3.4 g of viscous colorless liquid (3.4 g), yield 65.4%. Low resolution mass spectrum (LCMS) M+1=312.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com