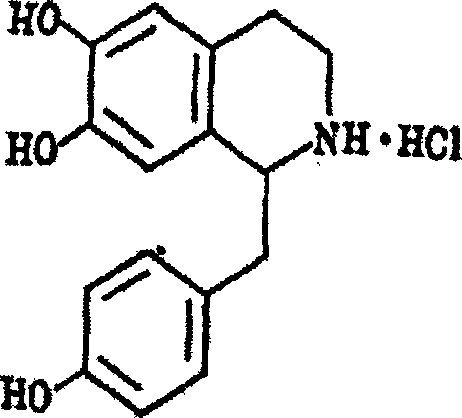
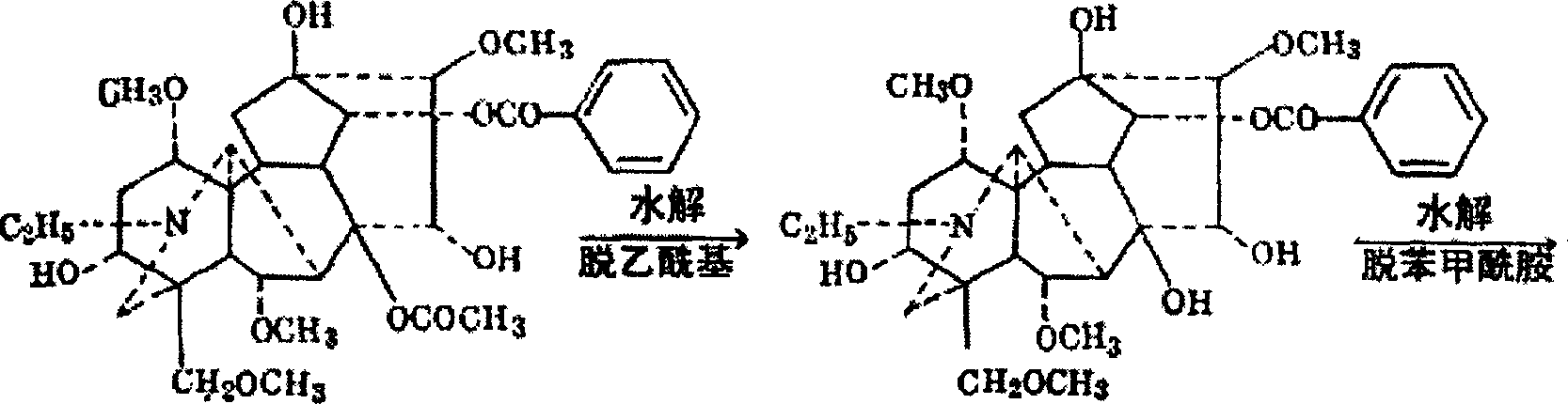
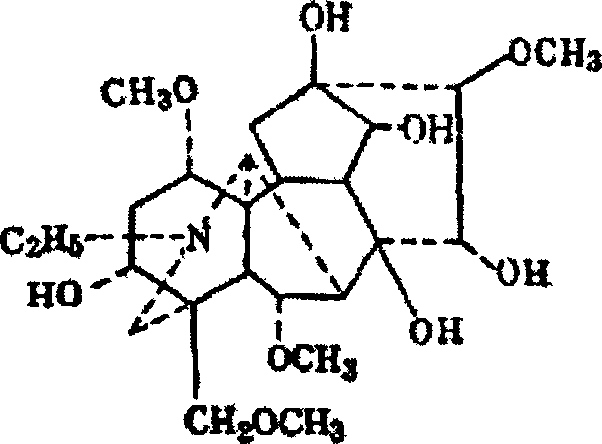
Use of aconine in medicine manufacture
A technology of aconitamine and analgesics, which is applied in the field of aconitamine hydrolyzed product of aconitine, can solve the problems of high toxicity, hidden danger, safety and the like of aconitine, so as to improve working efficiency, reduce toxicity and increase The effect of oxygen supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0033] Example 2 Acute toxicity test in mice
[0034] A. Test drug
[0035] Aconitine extract (1 gram is equivalent to 60 grams of native medicine), when used by humans, its concentration is formulated as 20mg / 100ml, 0.75ml / time, 3-6 times / day, calculated as 0.000015g / Kg per kilogram of body weight. day. The maximum concentration of the prepared aconitamine extract is 0.3g / ml, and it is used for gavage of mice during the experiment.
[0036] B. Test animals
[0037] Kunming mice were provided by the Experimental Animal Center of Sichuan Academy of Sciences and rationed with full-price pellets. After weighing, they were fed in boxes according to sex, 5 per box. The laboratory temperature is controlled at 18-22°C, and the humidity is controlled at 50-60%.
[0038] C. Test method
[0039] The method of administration is gastrointestinal administration, and a preliminary test is carried out before the formal experiment. The lethal dose range of aconitamine caused by 0% to 100% mortali...
Embodiment 3
[0045] Example 3 Hot plate analgesia test
[0046] Twenty-one female mice were selected and divided into 3 groups, 7 in each group. Different medicinal solutions were applied to the soles of the mice. The test group was smeared with aconitamine, the control group was smeared with morphine, and the blank group was smeared with saline. Heat the three iron plates to 50℃, let the three groups of mice move on the hot plate respectively, and measure the pain threshold (that is, the time to start licking the limbs and twisting the body) and the number of writhing times of the mice. The results are as follows :
[0047] Average pain threshold Number of twists
[0048] Test group 28 seconds 18 times
[0049] Control group 23 seconds 26 times
[0050] Blank group 12 seconds 34 times
[0051] It can be seen from the above results that not only there is a very significant difference between the test group and the blank group, P<0.005, but also between the test group and the control...
Embodiment 4
[0052] Example 4 Clinical experiment
[0053] Use aconitamine analgesic spray to spray the pain of 100 patients with gout; use aconitine analgesic ointment and / or cream to massage the pain of 500 patients with arthralgia and trigeminal neuralgia; use aconitamine Analgesic sprays or analgesic creams (creams) were used to treat 400 patients with low back and leg pain.
[0054] Results In the above cases, the pain relieved or disappeared within 3 minutes of medication.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com