Method for preparing medicinal mixture containing amoxicillin sodium and potassium clavulanate
A technology of potassium clavulanate and amoxicillin sodium, applied in the field of pharmaceutical preparation, can solve the problems of poor mixed powder fluidity, drug contamination, difficulty in aseptic sub-packaging, etc., and achieves good product fluidity, uniform particles, and good post-processing. performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
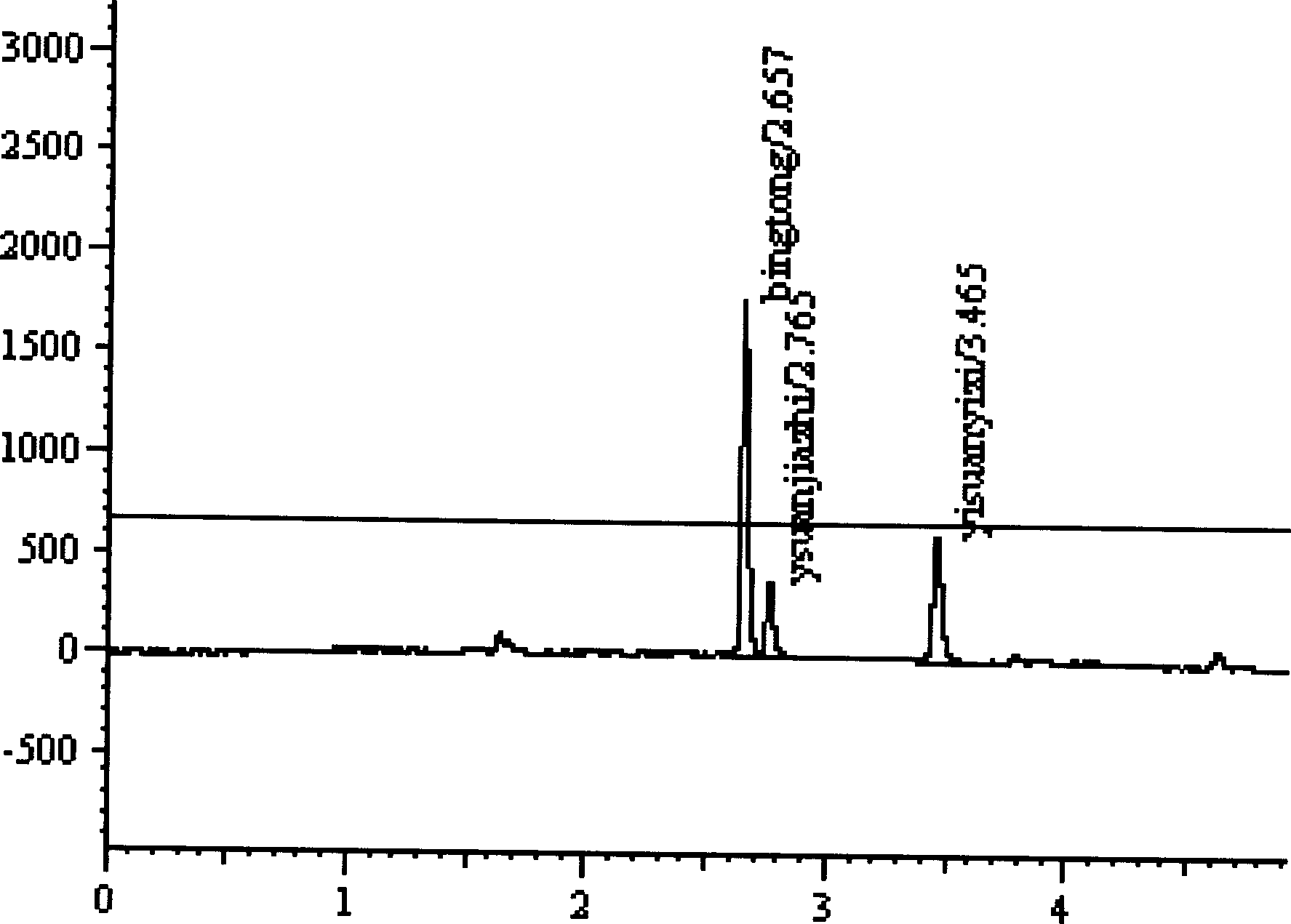
[0047] Amoxicillin sodium / potassium clavulanate was weighed in proportion and dissolved in methanol so that the concentrations of sodium amoxicillin and potassium clavulanate were 100 mg / ml and 20 mg / ml, respectively. After the CO2 gas is filtered, it is compressed to a predetermined pressure of 8.0MPa, and after preheating, it is pumped into the precipitator. The drug solution was sprayed into the supercritical fluid at a flow rate of 0.8ml / min, and the temperature of the precipitator was maintained at 30°C. After decompression, the obtained particles were collected at the bottom of the precipitator to obtain sample 1.
Embodiment 2
[0049] Weigh amoxicillin sodium / clavulanate potassium in proportion and dissolve them in distilled water, so that the concentrations of amoxicillin sodium and clavulanate potassium are 50 mg / ml and 5 mg / ml respectively. After the CO2 gas is filtered, it is compressed to a predetermined pressure range of 10MPa, and after preheating, it is pumped into the precipitator. The drug solution was sprayed into the supercritical fluid at a flow rate of 1.0ml / min, and the temperature of the precipitator was maintained at 35°C. After decompression, the obtained particles were collected at the bottom of the precipitator to obtain sample 2.
[0050] The supercritical fluid equipment used in the present invention is a commercially available product, such as the supercritical anti-solvent granulation system of Thar Technologies in the United States.
[0051] Take the same amoxicillin sodium and potassium clavulanate raw materials and mix them according to the conventional production method. ...
Embodiment 3
[0063] Amoxicillin sodium / potassium clavulanate was weighed in proportion and dissolved in a mixture of distilled water and methanol, so that the concentrations of amoxicillin sodium and potassium clavulanate were 100 mg / ml and 1 mg / ml, respectively. After the CO2 gas is filtered, it is compressed to a predetermined pressure range of 15MPa, and after preheating, it is pumped into the precipitator. The drug solution was sprayed into the supercritical fluid at a flow rate of 1.5ml / min, and the temperature of the precipitator was maintained at 40°C. After decompression, the resulting particles were collected at the bottom of the precipitator to obtain a sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com