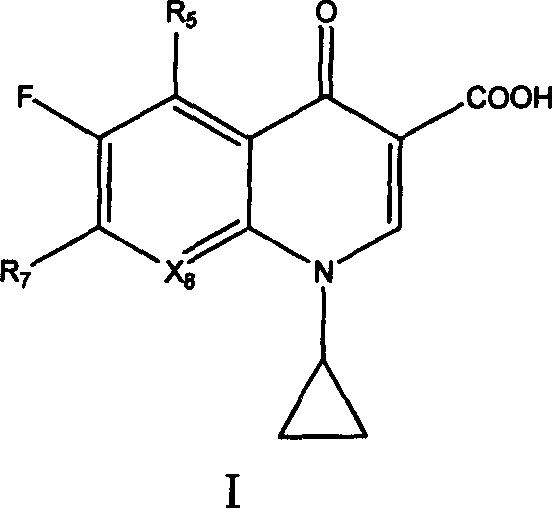
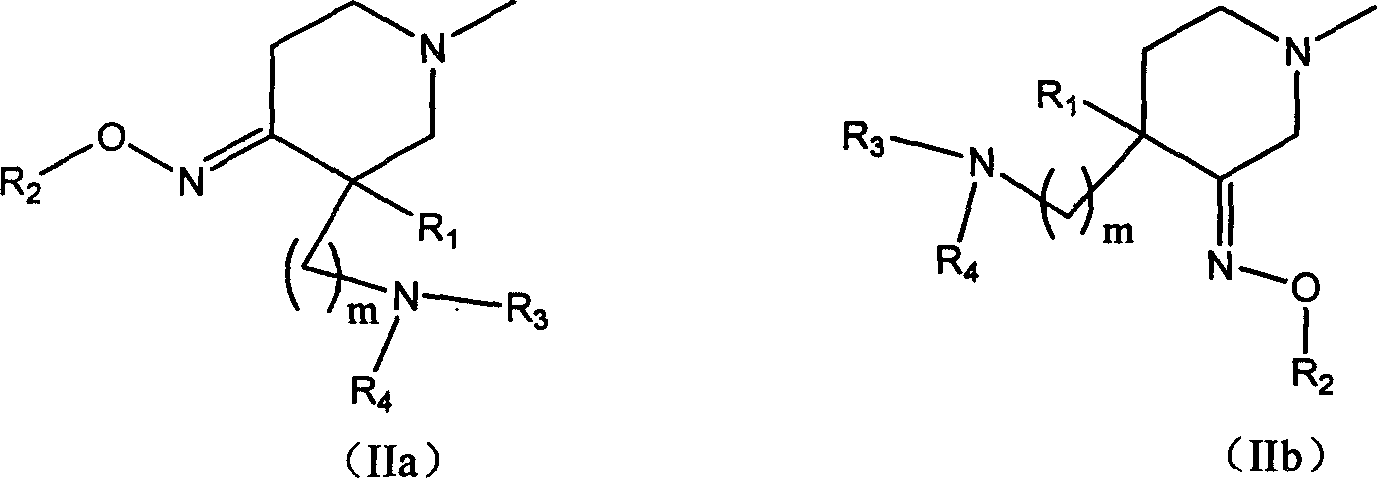
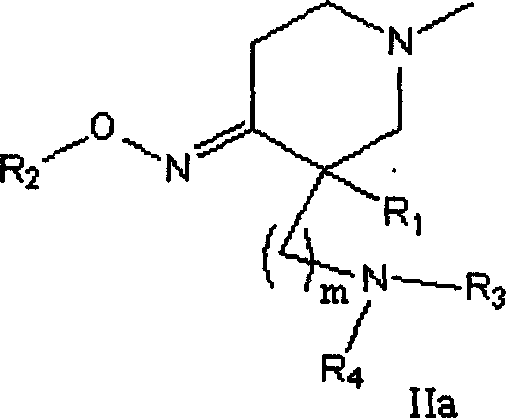
Quinolone compound containing oximino, and its preparing method and use
A compound, quinolone technology, applied in the fields of medicinal chemistry and chemotherapeutics, which can solve problems such as excluding piperidinyl groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0087] Synthesis of Methyl 1-(2-cyano-ethylamino)propionate
[0088]
[0089] Methyl 1-alanine (103.1 g 1 mol) (Tetrahedron. Lett. 42.21.2001.3525-3528) was dissolved in 300 ml of methanol, and acrylonitrile (63.7 g, 1.2 mol) was added. The reaction mixture was stirred under reflux for 5 hours, and the solvent was removed. The residue was distilled under reduced pressure (110-130°C / 10.0 Torr) to obtain 64.2 g (yield 42.0%) of the title compound. 1 H NMR (CDCl 3 , ppm): δ 3.59 (3H, s), 2.82 (4H, q), 2.42 (4H, t). MS (EI, m / e): 156 (M + )
preparation example 2
[0091] Synthesis of 3-cyano-1-(N-tert-butoxycarbonyl)-piperidin-4-one
[0092]
[0093]In the above formula and the following formulas, Boc represents tert-butoxycarbonyl. The compound (29.0 g, 0.186 mol) obtained in Preparation Example 1 was dissolved in 200 ml of chloroform, di-tert-butoxycarbonyl dicarbonate (45.0 g, 1.1 mol equivalent) was added thereto, and the reaction mixture was stirred at room temperature for 17 hours . The reaction solution was concentrated, and the residue was diluted with 250 ml of absolute ethanol. The resulting solution was added to a sodium ethoxide solution prepared by adding metallic sodium (Na) (10.7 g, 2.5 mol equivalents) to 220 ml of absolute ethanol under heating to reflux. After the addition was complete, the reaction was continued for an additional hour under reflux. The reaction solution was concentrated under reduced pressure, and the residue was diluted with water and washed with dichloromethane. The organic layer was separate...
preparation example 3
[0095] Synthesis of 3-methyl-3-cyano-1-(N-tert-butoxycarbonyl)-piperidin-4-one
[0096]
[0097] The compound (2.24 g, 10 mmol) prepared in Preparation Example 2 and tetrahydrofuran solution (12 ml, 1 mol / L, 1.2 mol equivalent) of tetra-n-butylammonium fluoride (TBAF) were dissolved in 100 ml of tetrahydrofuran. To which was added iodomethane (CH 3 I) (2.82 g, 2 mol equivalents). The reaction mixture was stirred overnight at room temperature. The reaction solution was concentrated, and the residue was dissolved in 200 ml of ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, and filtered. The filtrate was concentrated, and the crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:10) to obtain 1.49 g (yield 63.0%) of the title compound. 1 H NMR (CDCl 3 , ppm): δ4.2 (2H, m), 3.4-2.5 (4H, m), 1.45 (9H, s), 1.4 (3H, s). MS (EI, m / e): 238 (M + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com