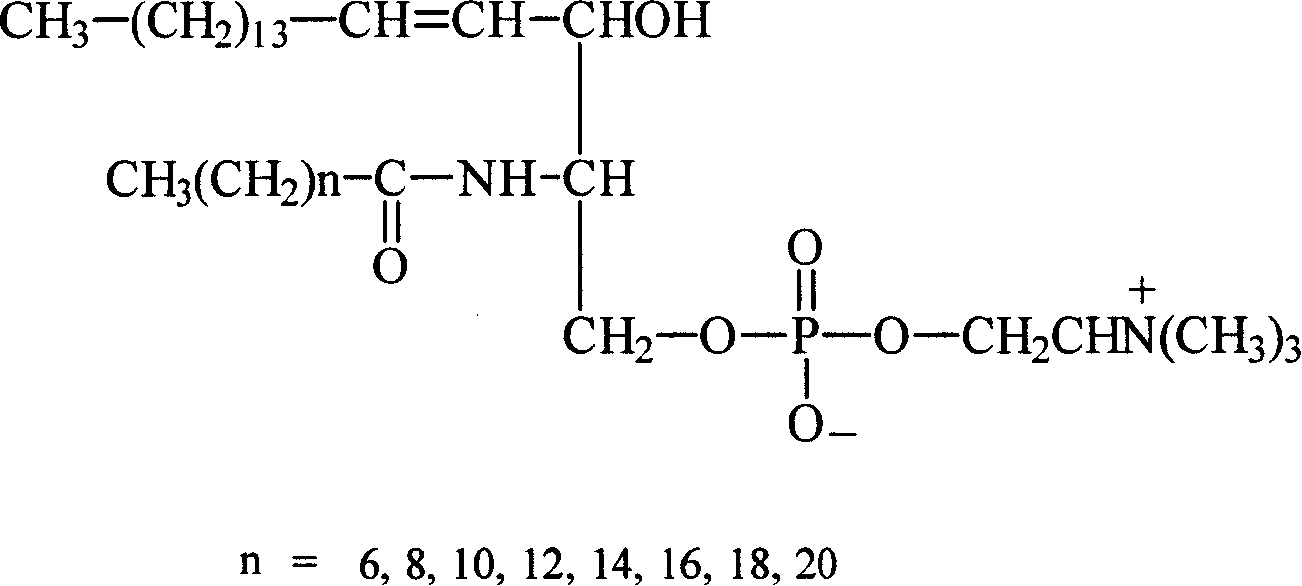
Prepn. of phosphosphingolipid isopropylphenol fatty emulsion and its prepn. method
A technology of propofol fat and propofol fat, which is applied in the formulation and preparation of propofol sphingomyelin fat emulsion preparations, can solve problems affecting the stability of pharmaceutical preparations, clinical safety use restrictions, pain and inflammation, and achieve Extend the retention time, solve the stability and reduce the effect of leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: low propofol content sphingomyelin propofol fat emulsion preparation (0.1%, weight content)
[0028] Propofol
[0029] Preparation method: Take 1.0g of propofol, 3.0g of vitamin E, and 196.0g of refined soybean oil, add 6.0g of sphingomyelin and 6.0g of lecithin respectively, heat and stir for about 10-20 minutes and mix well. Another 700ml of water for injection was taken, and 22.0g of glycerin was added. Under the condition of nitrogen protection and stirring, the propofol sphingomyelin oil solution was added to the aqueous glycerin solution, and the total amount was adjusted to 1000 ml. Homogenize 7-8 times with a high-pressure homogenizer, the homogenization pressure is 100MPa, until the particle size ranges from 180nm to 300nm, adjust the pH to 7.0-8.0, filter, sub-package, inject nitrogen, and seal. Sterilize at 115°C for 30 minutes, after passing the light inspection, pack and store below 25°C.
Embodiment 2
[0030] Embodiment 2: high propofol content sphingomyelin propofol fat emulsion preparation (8%, weight content)
[0031] Propofol
80.0g
soybean oil (for injection)
117.0g
Sphingomyelin (for injection)
6.0g
Lecithin (for injection)
6.0g
Vitamin E
3.0g
Glycerin
22.0g
Water for Injection
Add to 1000ml
[0032] Preparation method: Take 80.0g of propofol, 3.0g of vitamin E and 117.0g of refined soybean oil, add 6.0g of sphingomyelin and 6.0g of lecithin respectively, heat and stir for about 10-20min and mix well. Another 700ml of water for injection was taken, and 22.0g of glycerin was added. Under the condition of nitrogen protection and stirring, the propofol sphingomyelin oil solution was added to the aqueous glycerin solution, and the total amount was adjusted to 1000 ml. Homogenize 7-8 times with a high-pressure homogenizer, the homogenization pressure is 100MPa, until t...
Embodiment 3
[0036] Embodiment 3: all adopt the sphingomyelin propofol fat emulsion preparation prepared by sphingomyelin
[0037] Propofol
[0038] Preparation method: Take 10.0g of propofol, 3.0g of vitamin E, 187.0g of refined soybean oil, add 12.0g of sphingomyelin, heat and stir for about 10-20min to mix well. Another 700ml of water for injection was taken, and 22.0g of glycerin was added. Under the condition of nitrogen protection and stirring, the propofol sphingomyelin oil solution was added to the aqueous glycerin solution, and the total amount was adjusted to 1000 ml. Homogenize 7-8 times with a high-pressure homogenizer, the homogenization pressure is 100MPa, until the particle size ranges from 180nm-300nm, adjust the pH to 7.0-8.0, filter, subpackage, inject nitrogen, and seal. Sterilize at 115°C for 30 minutes, and pack after passing the light inspection. Store below 25°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com