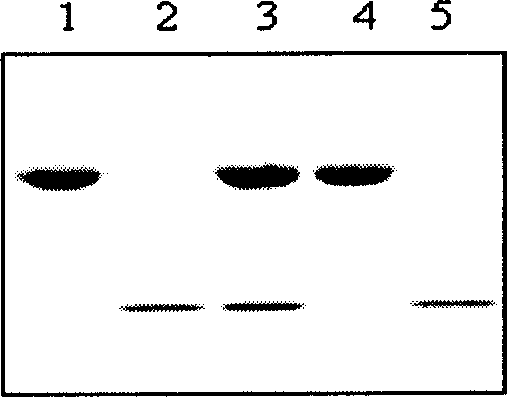
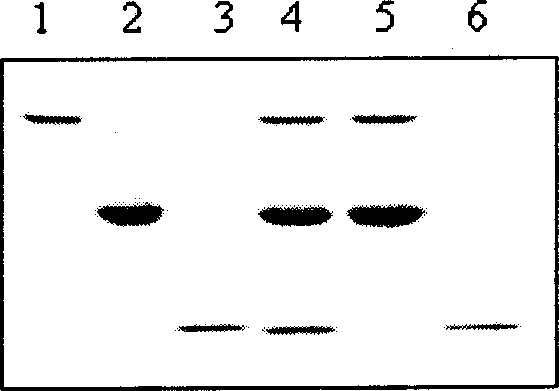
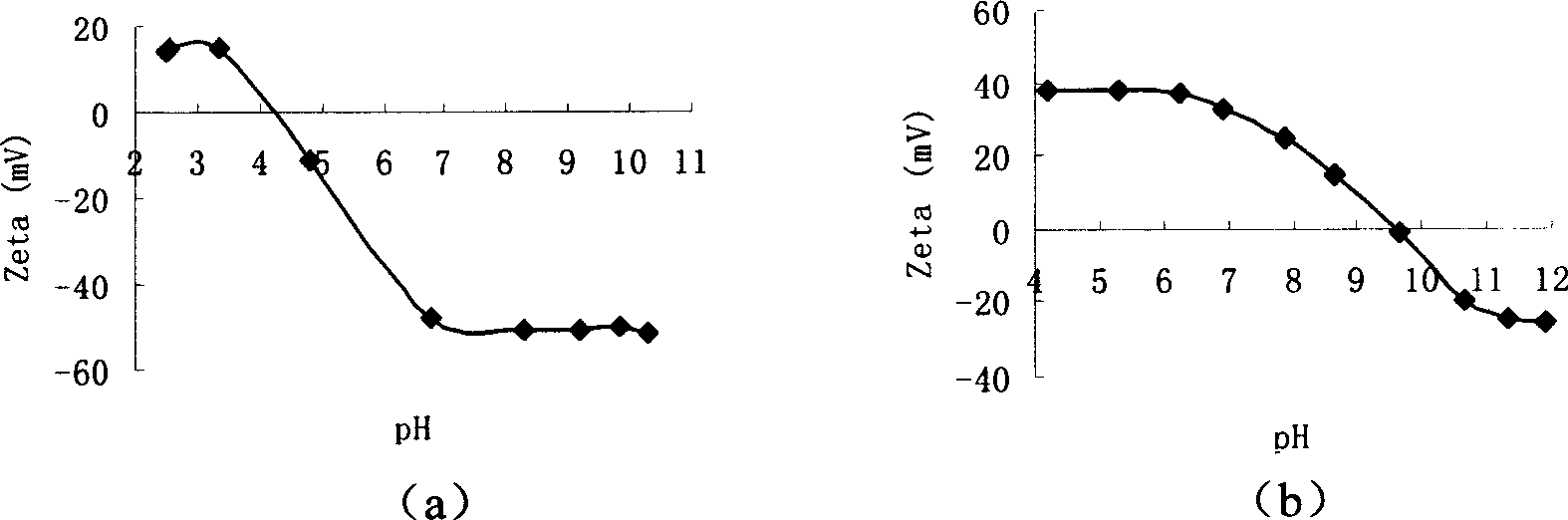Method for separating protein adopting superparamagnetism silicon nanometer granule static adsorption
A superparamagnetic and nanoparticle technology, which is applied in the application field of nanomaterials and nanobiology, can solve the problems of cumbersome pretreatment of ion exchange columns and non-specific adsorption of proteins, so as to improve the action speed and reduce the operation intensity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the extraction of cytochrome C in the mixed solution of bovine serum albumin (acidic protein, pI4.6) and cytochrome C (basic protein, pI10.6):
[0036] 1. Adjustment of the pH value of the mixed solution of cytochrome C and bovine serum albumin: at room temperature, use 1 / 15 mol / L disodium hydrogen phosphate solution to adjust the pH value of the mixed protein solution to 8.0.
[0037] 2. Formation of superparamagnetic pure silicon shell nanoparticles-cytochrome C complex: Add 15mg superparamagnetic pure silicon shell nanoparticles to 5.0mL mixed protein solution, mix well and After incubation for 0.50 hours, a complex of superparamagnetic pure silicon shell nanoparticles-cytochrome C is formed.
[0038] 3. Separation and collection of superparamagnetic pure silicon shell nanoparticles-cytochrome C complex: using the superparamagnetism of superparamagnetic pure silicon shell nanoparticles, under the action of an external magnetic field, the superparamagnet...
Embodiment 2
[0042] Example 2: Cytochrome C in a mixed solution of bovine serum albumin (acidic protein, pI4.6), immunoglobulin G (basic protein, pI8.0) and cytochrome C (basic protein, pI10.6) Extraction of:
[0043] 1. Adjustment of the pH value of the mixed solution of bovine serum albumin, immunoglobulin G and cytochrome C: at room temperature, use 1 / 15 mol / L disodium hydrogen phosphate solution to adjust the pH value of the mixed protein solution to 8.0.
[0044] 2. Formation of superparamagnetic pure silicon shell nanoparticles-cytochrome C complex: Add 3.0mg superparamagnetic pure silicon shell nanoparticles to 1.0mL mixed protein solution, mix thoroughly at room temperature of 15-25°C and After incubation for 0.50 hours, a complex of superparamagnetic pure silicon shell nanoparticles-cytochrome C is formed.
[0045] 3. Separation and collection of superparamagnetic pure silicon shell nanoparticles-cytochrome C complex: using the superparamagnetism of superparamagnetic pure silicon...
Embodiment 3
[0049] Embodiment 3: the extraction of bovine serum albumin in the mixed solution of bovine serum albumin (acidic protein, pI4.6) and cytochrome C (basic protein, pI10.6):
[0050] 1. Adjustment of the pH value of the mixed solution of cytochrome C and bovine serum albumin: at room temperature, use 1 / 15 mol / L potassium dihydrogen phosphate solution to adjust the pH value of the mixed protein solution to 5.0.
[0051] 2. Formation of superparamagnetic aminated silicon shell nanoparticles-bovine serum albumin complex: Add 9.0 mg of superparamagnetic aminated silicon shell nanoparticles to 3.0 mL of mixed protein solution, at room temperature of 15-25 °C, Mix well and incubate for 0.50 hour to form a superparamagnetic aminated silicon shell nanoparticle-bovine serum albumin complex.
[0052] 3. Separation and collection of superparamagnetic aminated silicon shell nanoparticles-bovine serum albumin complex: use the superparamagnetism of superparamagnetic aminated silicon shell nan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com