Transdermal drug administration intensifier and its usage method
A technology for transdermal drug delivery and enhancer, which is used in pharmaceutical formulations, preparations for skin care, botanical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Example 1 , In vivo phage display technology to screen peptides with skin penetration enhancing function
[0115] The library used to screen peptides with transdermal enhancing function was provided by New England Biolabs (Mass, MA), a library of heptapeptides containing disulfide bonds among three random peptide libraries (Ph.D.-C7C). Random fragments in this library are flanked by cysteine residues that can be oxidized during phage assembly to form disulfide linkages, thus forming a cyclic peptide that interacts with the target. (Ph.D. TM -C7C Phage Peptide Display Kit [online], [purchased on 2005-09-01]. I learned the information on the Internet
[0116] http: / / www.neb.com / nebecomm / products / productE8120.asp >). This library contains more than two billion clones. Random peptides in the library are amino-terminal to the small coat protein pill, so five copies are expressed per phage particle. The position of the phage-expressed random sequence in the Ph.D.-C7C...
Embodiment 2
[0119] Example 2 , chemical synthesis of transdermal enhancers and their analogues
[0120] The peptides in this patent are all synthesized by Shanghai Jier Biochemical Company using the standard FMOC solid-phase synthesis method in an automatic peptide synthesizer (CS536-1381, CS Bio Co., Menlo Park, CA), and purified by HPLC to make the purity greater than 95 %, using a mass spectrometer (BIFLEX TM III, Bruker, Germany) to identify molecular weights.
[0121] The peptide synthesis procedure of the sequence ACSSSPSKHCG (TD-1, SEQ ID NO: 2) is as follows. The terminal amino acids alanine and glycine are derived from the M13 surface protein. TD-1 uses the standard FMOC solid-phase synthesis method, and the general manual synthesis steps are as follows: about 0.2 mmol Fmoc-Gly-Wang resin is added to the manual reaction tube (Peptides International), and DMF is added to allow it to expand for 2 hours. Then add 20% piperidine / DMF to react for 2min to remove the Fmoc protecti...
Embodiment 3
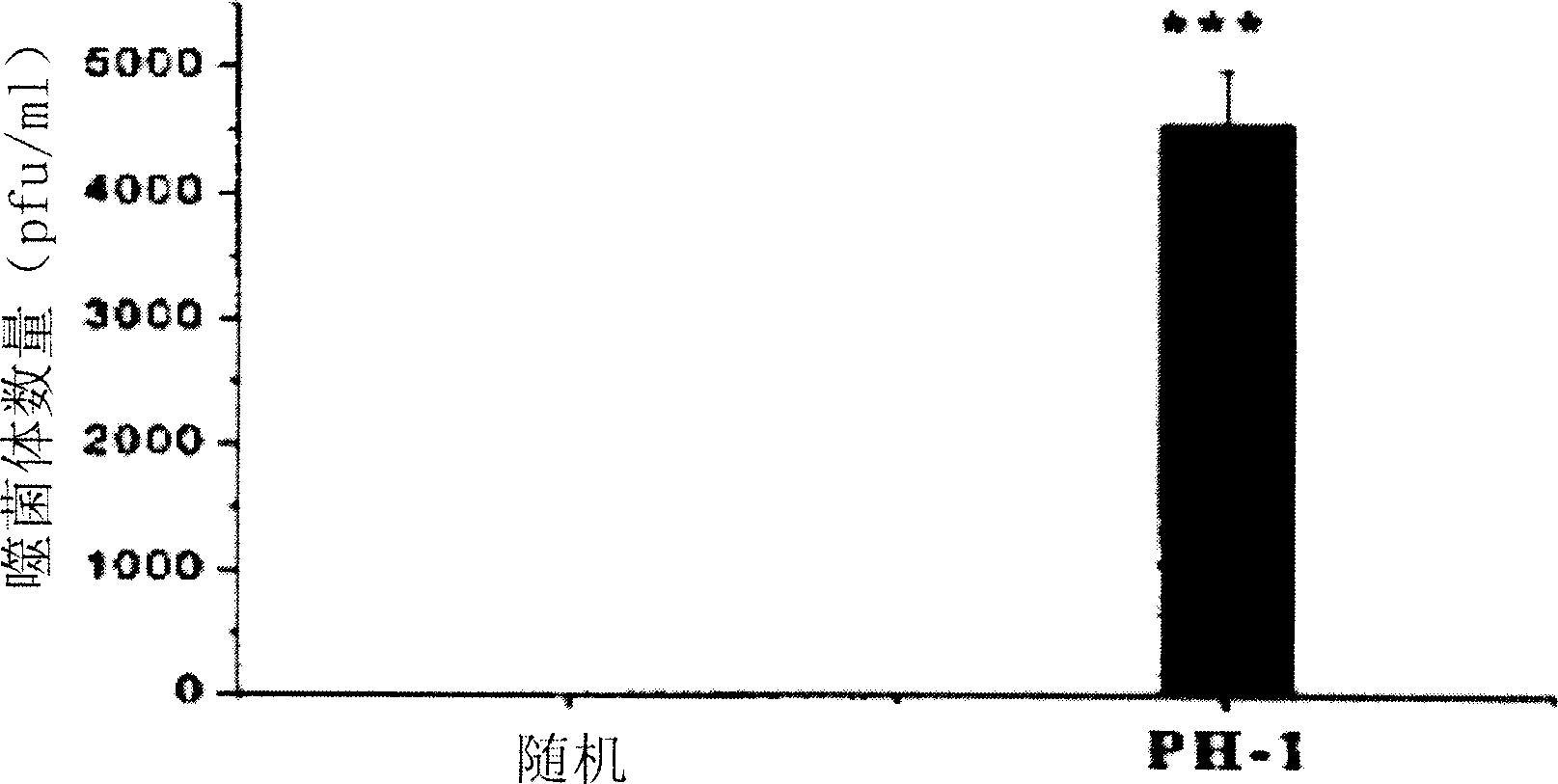
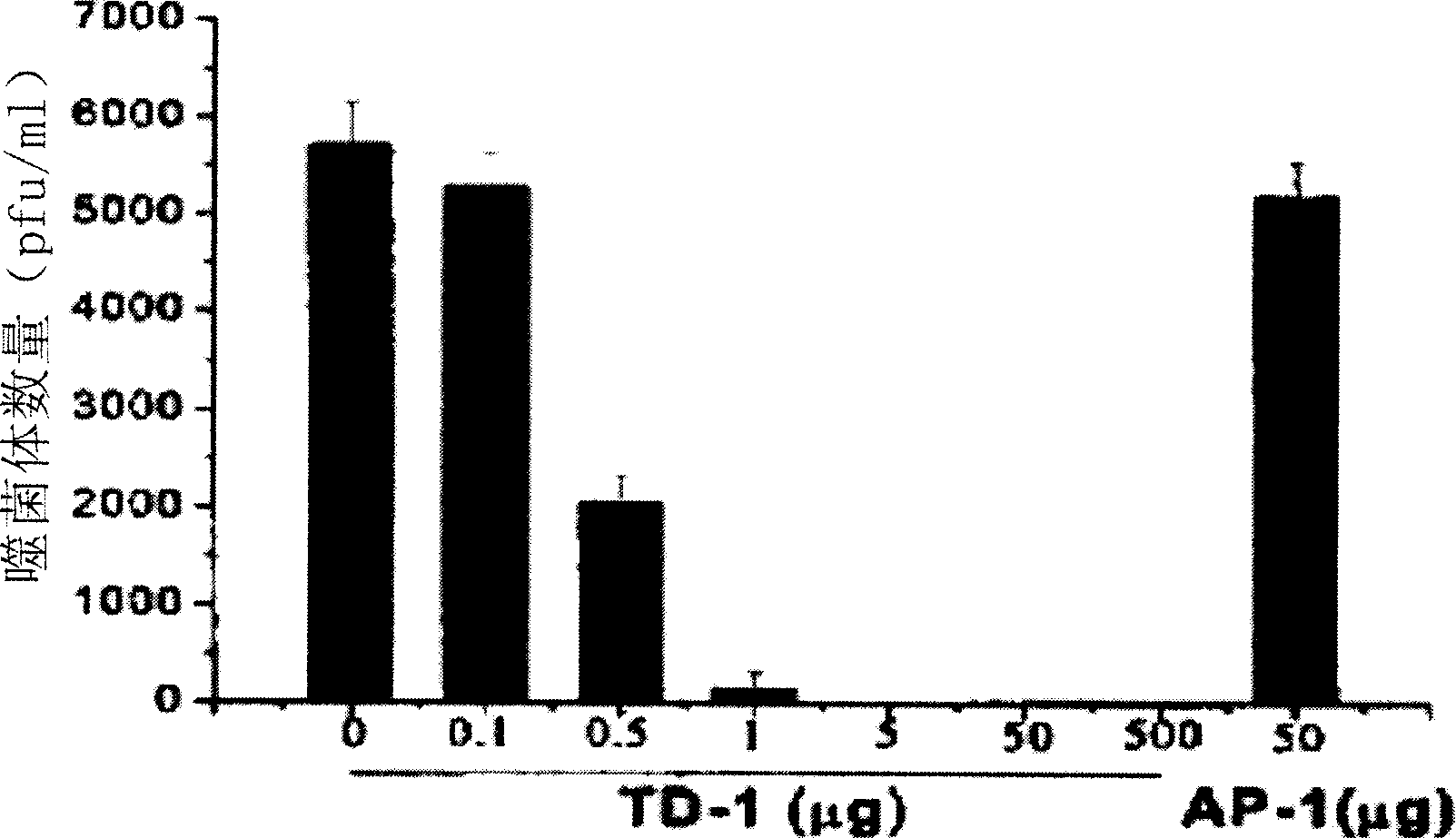
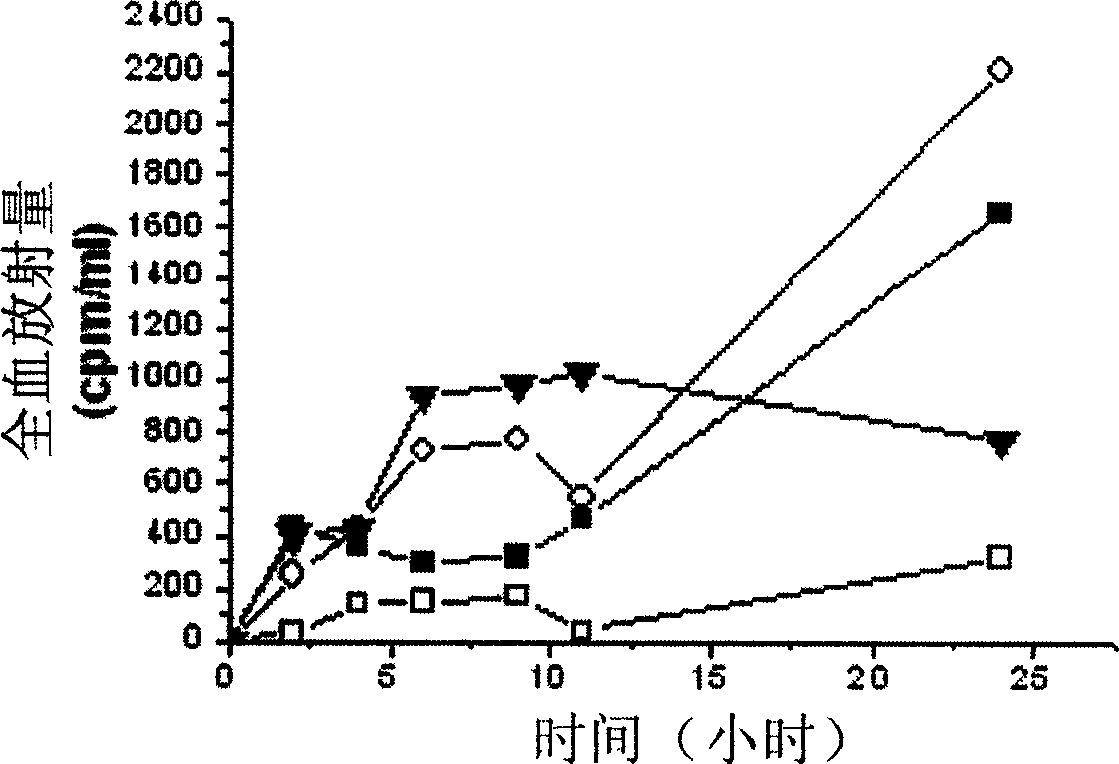
[0123] Example 3 , TD-1 inhibits the transdermal activity of PH-1
[0124] Male Wistar rats (180 g to 220 g) were housed in animal rooms with constant temperature (22°C), constant relative humidity (60%), and a fixed 12-h light / dark cycle, and they were free to take food and water. Anesthetize the mice with molasses (5 mg / kg, concentration 20%), and then use scissors to cut off the hair in the abdomen area of about 3 cm × cm, taking care not to damage the skin (the mice with any visible scars are discarded). Display of TD-2 peptide (SEQ ID NO: 1) 10 12 PH-1 phage or control phage, the control phage is randomly picked from the library, and the coding sequence AP-1 (sequence number: 6), is placed in the shearing area, and spreads it on the whole area with the side of the pipette tip . One hour later, blood was drawn from the tail vein and titrated according to the manufacturer's instructions.
[0125] Tested in Wistar rats, it was shown that phages expressing TD-1 pepti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com