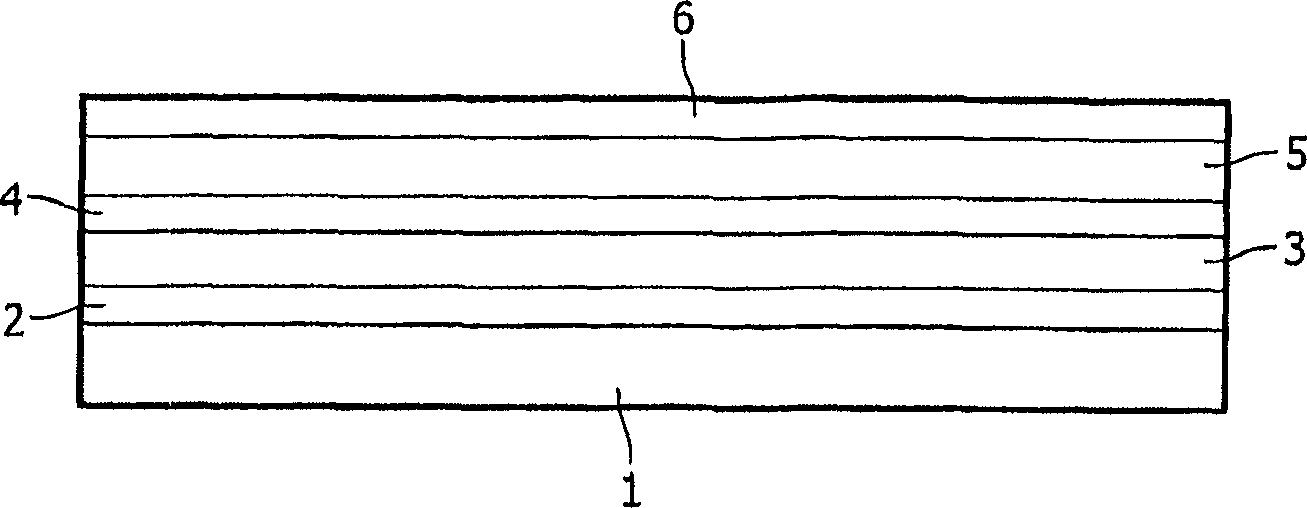
Light-emitting device with an iridium complex
A technology of light-emitting devices and iridium complexes, which can be applied to indium organic compounds, platinum group organic compounds, and light-emitting materials, and can solve problems such as decreased eye sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0053] Dichloro-bridged dimer (dbzq) 2 Ir(μ-Cl) 2 (dbzq) 2 Synthesis
[0054] 250mg of IrCl 3 ·3H 2 O and 407 mg of dibenzo[f,h]quinoline were dissolved in a mixture of 2-ethoxyethanol (20 ml) and water (7 ml) and heated at reflux for 24 hours. After cooling to room temperature, the resulting yellow precipitate was centrifuged, washed with ethanol (60ml) and acetone (60ml) and dried.
[0055] 370 mg (77%) of the dichloro-bridged dimer were obtained in the form of a yellow powder.
Embodiment approach 2
[0057] Starts with (dbzq) 2 Ir(μ-Cl) 2 (dbzq) 2 to synthesize Ir(dbzq) 2 (acac).
[0058] Will (dbzq) 2 Ir(μ-Cl) 2 (dbzq) 2 Heat and reflux in 2-ethoxyethanol with 2.5 equivalents of acetylacetonate and 400 mg of sodium carbonate for 20 hours. The resulting orange precipitate was centrifuged and washed with water, n-hexane, diethyl ether and ethanol. The yield of crude product was 70-75%.
[0059] The crude product was purified by column chromatography on silica gel (CH 2 Cl 2 / n-hexane / diethyl ether).
[0060] 1 H-NMR (CDCl 3 ): δ=1.80(s, 6H, 2CH 3 ), 5.29 (s, 1H, CH), 6.33 (d, J=7.3Hz, 2H, CH arom.), 6.97 (t, 2H, CH arom.), 7.61 (dd, J=7.7Hz, J=5.4 Hz, 2H, CH arom.), 7.66 to 7.72 (m, 4H, CH arom.), 7.86 (d, J=8.0Hz, 2H, CH arom.), 8.56 to 8.60 (m, 2H, CH arom.), 8.64 to 8.67 (m, 2H, CH arom.), 8.87 to 8.91 (m, 4H, CH arom.).
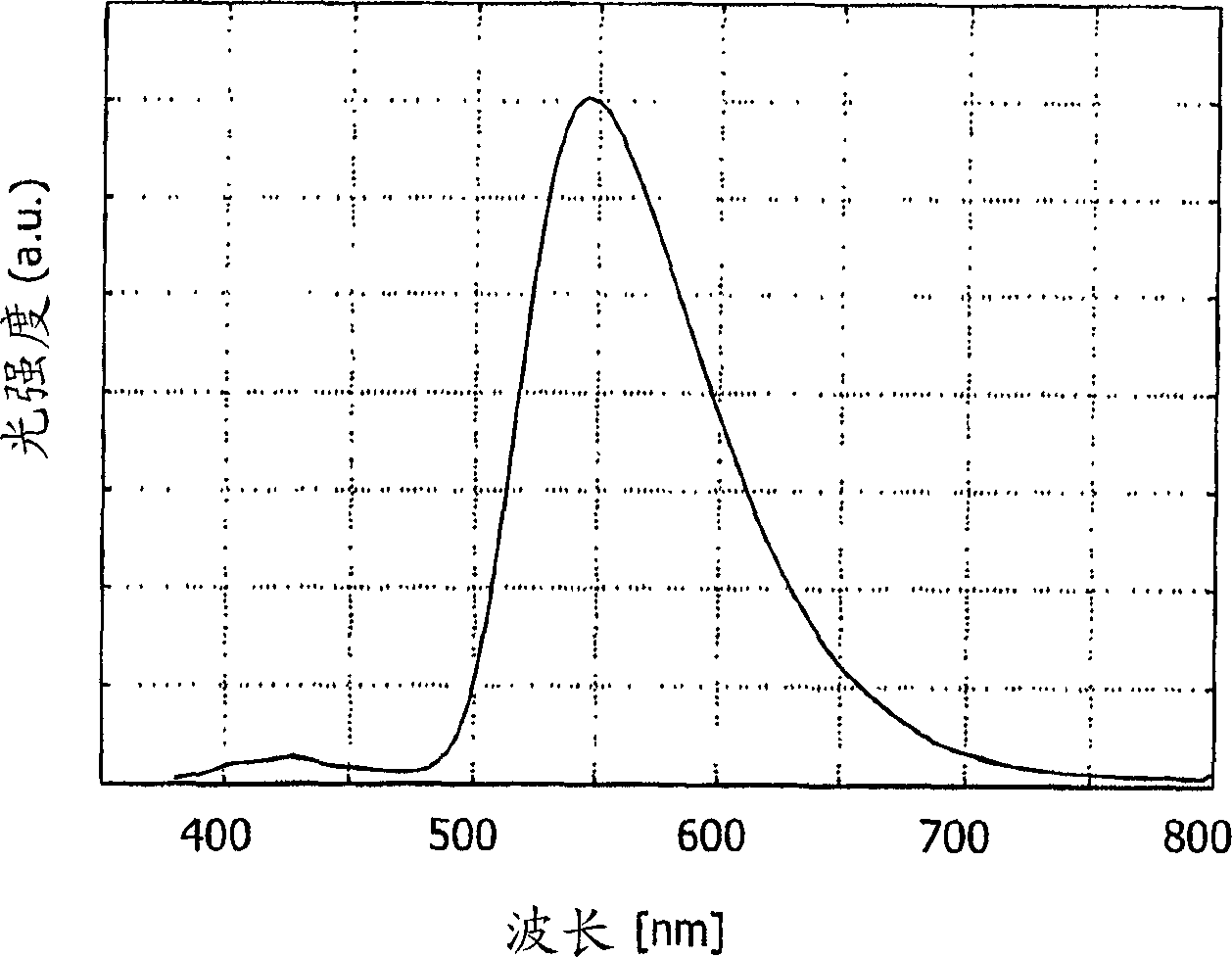
[0061] The obtained complex Ir(dbzq) 2 (acac) emission wavelength λ max is light at 545 nm (in methyl THF). The emission spectrum i...
Embodiment approach 3
[0063] Ir(dbzq) 3 Synthesis
[0064] 53 mg of iridium(III) acetylacetonate and 250 mg of dibenzo[f,h]quinoline were added to 5 ml of degassed ethylene glycol. The resulting suspension was heated and refluxed for 60 hours. After cooling to room temperature, the reaction mixture was added to 10 ml of 1N HCl. After stirring for 5 minutes, the orange crystalline precipitate was filtered off, rinsed with 5 ml of 1N HCl and water and dried.
[0065] The product was dissolved in dichloromethane, filtered through silica gel and dried.
[0066] 1 The H-NMR spectrum shows that the product contains both fa- and meridian-isomers of the complex, where the meridian-isomer has a greater proportion.
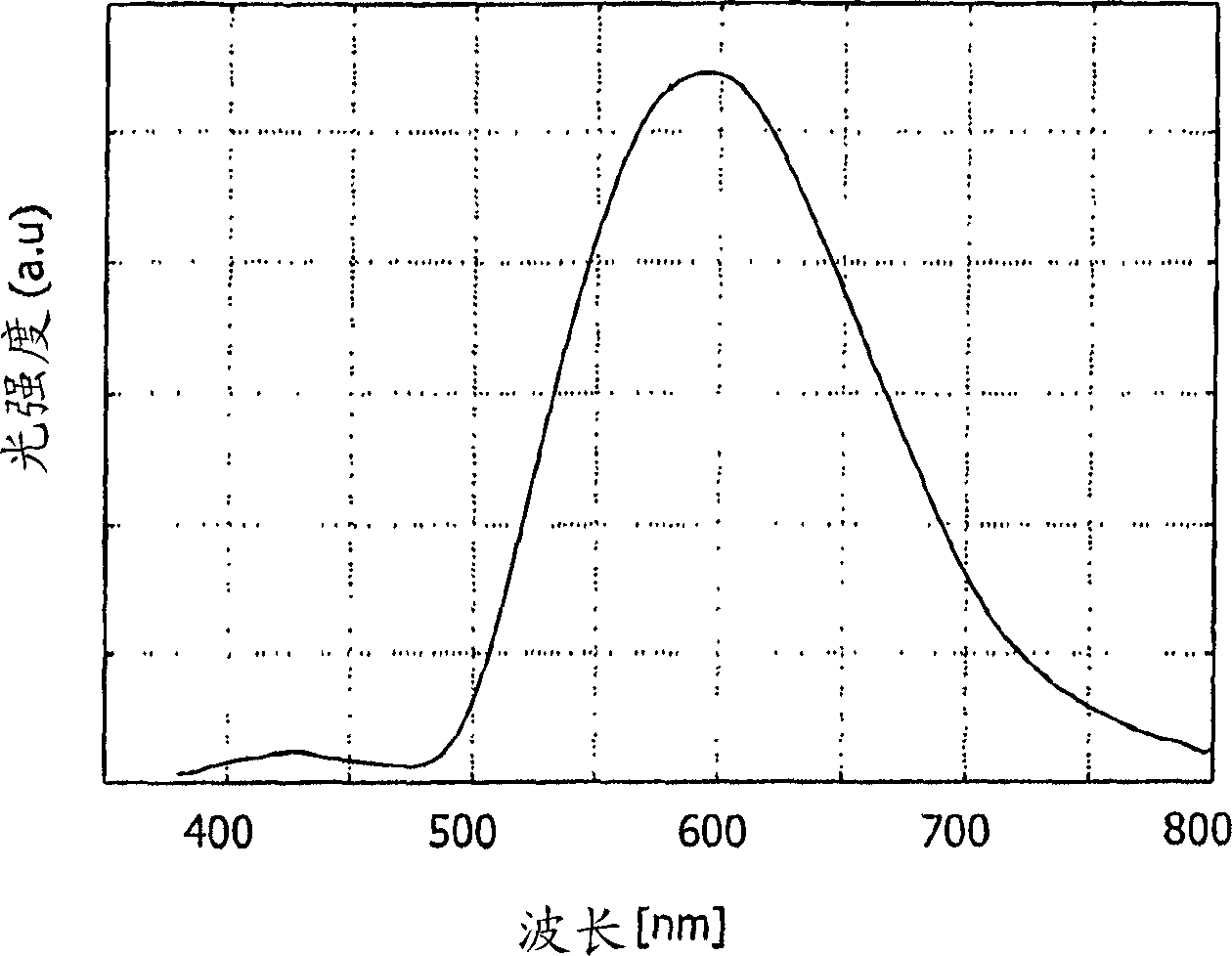
[0067] The face / mer-isomer mixture in the complex emits at wavelength λ max is light at 595 nm (in methyl THF). The emission spectrum is shown in image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com