Quality control method for medicinal composition containing artemisine
A composition and technology of artemisinin, applied in the field of medicine, can solve the problems of affecting the test results, many impurities, poor stability, reproducibility and recovery rate, etc., and achieve the effect of strong controllability and simplified operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
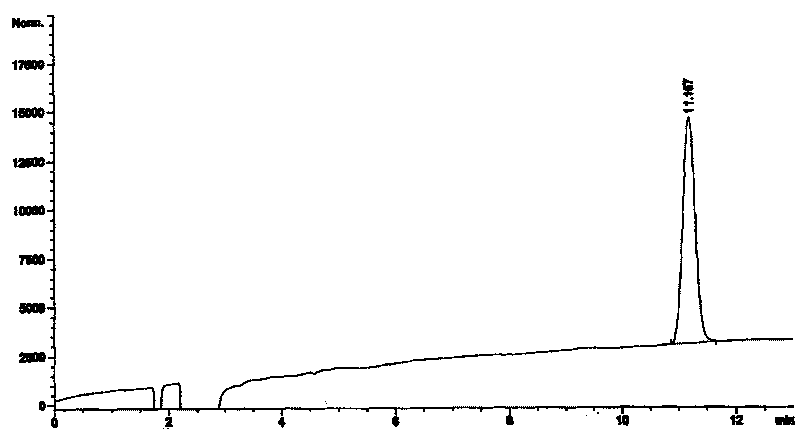
[0034] The HPLC collection of illustrative plates of embodiment 1 artemisinin of the present invention
[0035] 1. Detection method:
[0036] (1) Main instruments and reagents: HP1100 liquid chromatograph
[0037] Chromatographic methanol is chromatographically pure, chromatographic water is double-distilled water, and the rest are analytically pure.
[0038] (2) Instrument conditions
[0039] Detector: differential detector
[0040] Mobile phase: methanol-water (72:28)
[0041] Flow rate: 1.0ml / min
[0042] Column temperature: 30°C
[0043] Column: Kromasil KR100-5C18 250*4.6mm E17580
[0044] Injection volume: 20ul
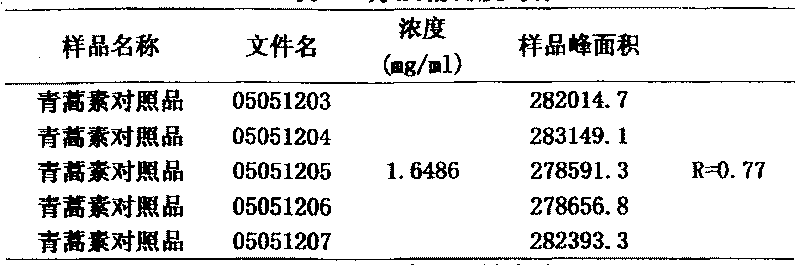
[0045](3) Standard curve drawing: Weigh 82.43 mg of artemisinin reference substance into a 50 ml volumetric flask, dissolve in 95% methanol and make to volume. Pipette 0.5ml, 1ml, 3ml, 5ml, and 10ml of the prepared artemisinin reference solution into 10ml volumetric flasks, add 95% methanol to dilute to the mark, and inject samples to obtain the artemisi...
Embodiment 2
[0056] Embodiment 2: the HPLC collection of illustrative plates of artemisinin reference substance of the present invention
[0057] The chromatographic conditions are: detector: differential detector, mobile phase: methanol-water volume ratio of 72:28, flow rate: 1.0ml / min, chromatographic column: Kromasil KR100-5C18 250*4.6mm E17580 lower artemisinin reference substance The chromatogram, see Figure 1.
Embodiment 3
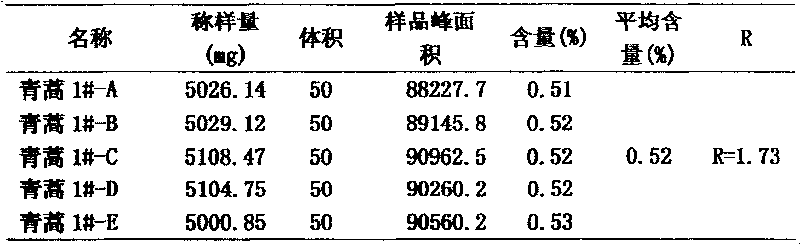
[0058] The quality control of embodiment 3 Artemisia annua of the present invention
[0059] Evenly take about 500g of Artemisia annua, crush it through a 60-mesh sieve. Precisely weigh 5.10847g of crushed medicinal material in a 250ml flat-bottomed flask, extract 3 times at 60°C, use 100ml n-hexane each time, extraction time is 2h, 1.5h, 1.5h, filter with suction, wash the residue three times with a little n-hexane , Combine the filtrate and washing liquid, concentrate the film at 55±5℃ until it is nearly dry, dissolve the concentrated residue with 95% methanol, fully dissolve it and put it into a 50ml volumetric flask, dilute to volume with 95% methanol, shake well, 0.45um Membrane filtration.
[0060] The quality control method is the same as in Example 1, and the chromatographic conditions are: detector: differential detector, mobile phase: the volume ratio of methanol-water is 72:28, flow rate: 1.0ml / min, chromatographic column: Kromasil KR100-5C18 250*4.6 The chromatog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com