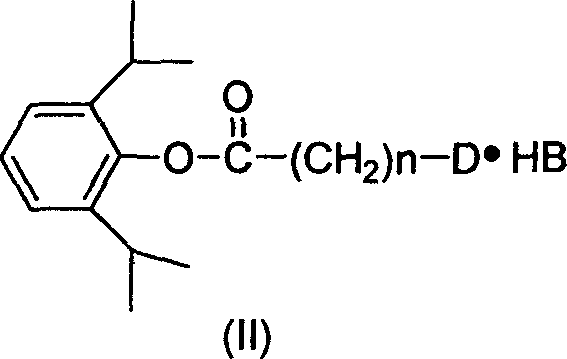
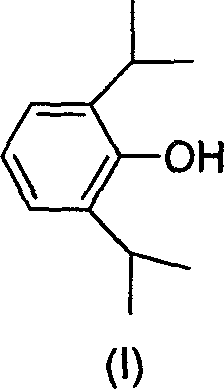
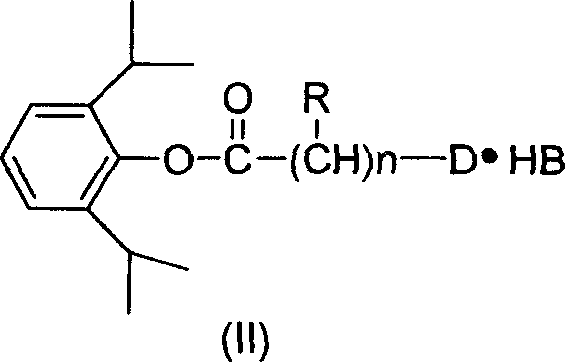Water-soluble derivative of anesthetic 2,6-diisopropyl phenol and preparation method thereof
A technology of diisopropylphenyl and derivatives, applied in anesthetics, drug combinations, pharmaceutical formulations, etc., can solve problems such as poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of 2,6-diisopropylphenyl-2-chloroacetate from chloroacetyl chloride:
[0024] Under the flow of dry N2, 30 ml of anhydrous ether, 5.13 g of 2,6-diisopropylphenol, and 3.0 ml of chloroacetyl chloride were added to a 100 ml dry three-necked flask. Cool down to about -5°C; slowly add 5g of pyridine. Control the internal temperature at -5°C ± 2°C. After the dropwise addition, the ice-salt bath was removed, and the temperature was naturally raised at room temperature, and the reaction was continued at room temperature for 4 hours until the reaction was complete. Suction filtration, the filtrate was washed with ice-brine until the water layer was neutral, and then dried with anhydrous Na2SO4; suction filtration, the filtrate was concentrated under reduced pressure, and diethyl ether was evaporated to obtain 7.2 g of oil, with a yield of 95%.
Embodiment 2
[0026] Preparation of 2,6-diisopropylphenyl-2-chloroacetate from chloroacetic acid:
[0027] Under the flow of dry N2, 30 ml of dichloromethane, 5.13 g of 2,6-diisopropylphenol, and 2.8 g of chloroacetic acid were added to a 100 ml dry three-necked flask. Appropriate amount of DCC. React at room temperature for more than 3 hours, add 10 ml of dilute hydrochloric acid and stir for more than 1 hour, separate the water layer, and evaporate the solvent under reduced pressure to obtain 6.5 g of oil, with a yield of 88%.
Embodiment 3
[0029] Preparation of N,N-2,6-diisopropylphenyl-2-dimethylaminoacetate hydrochloride from 2,6-diisopropylphenyl-2-chloroacetate
[0030] Dissolve 7.2g of 2,6-diisopropylphenyl-2-chloroacetate in 50ml of acetone, under stirring, pass through dry dimethylamine gas, and react to the end; concentrate under reduced pressure, add 20ml of ether to dissolve the oil The liquid was washed with 10ml of saturated sodium bicarbonate solution, and then washed with saturated ice brine until the water layer was neutral; the organic layer was dried with anhydrous Na2SO4, filtered with suction, stirred, and slowly fed with dry hydrogen chloride gas, filtered with suction , to obtain 6.8g of white solid, yield 80%; take 0.66g of crude product, add 45ml of acetone, a small amount of activated carbon is stirred and heated to reflux for decolorization, filter, add an appropriate amount of ether, freeze crystallization, suction filter, wash with a small amount of ether, and place in an oven at 50°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com