Fire-retardancy styrene resin composition and its forming product
A technology of styrene-based resin and resin composition, which is applied in the field of flame-retardant styrene-based resin composition, and can solve problems such as polluting molds, extreme decline in heat resistance, and damage to the characteristics of styrene-based resins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
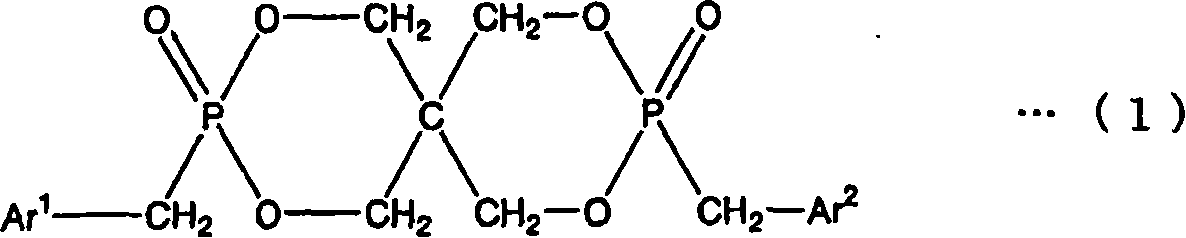
[0193] Preparation of 2,4,8,10-tetraoxa-3,9-diphosphospiro[5.5]undecane, 3,9-dibenzyl-3,9-dioxide (FR-1)
[0194] 816.9 g (6.0 mol) of pentaerythritol, 19.0 g (0.24 mol) of pyridine, and 2250.4 g (24.4 mol) of toluene were added and stirred in the reaction container provided with the thermometer, the condenser, and the dropping funnel. 1651.8 g (12.0 mol) of phosphorus trichloride was added to this reaction container using this dropping funnel, and it heat-stirred at 60 degreeC after completion|finish of addition. After the reaction, it was cooled to room temperature, 26.50 parts of methylene chloride was added to the obtained reactant, and 889.4 g (12.0 mol) of t-butanol and 150.2 g (1.77 mol) of methylene chloride were added dropwise while ice-cooling. The resulting crystals were washed with toluene and dichloromethane and filtered. At 80°C, 1.33×10 2 The obtained filtrate was dried under Pa for 12 hours to obtain 1341.1 g (5.88 mol) of a white solid. pass 31 P. 1 HNMR ...
preparation example 2
[0199] Preparation of 2,4,8,10-tetraoxa-3,9-diphosphospiro[5.5]undecane, 3,9-dibenzyl-3,9-dioxide (FR-2)
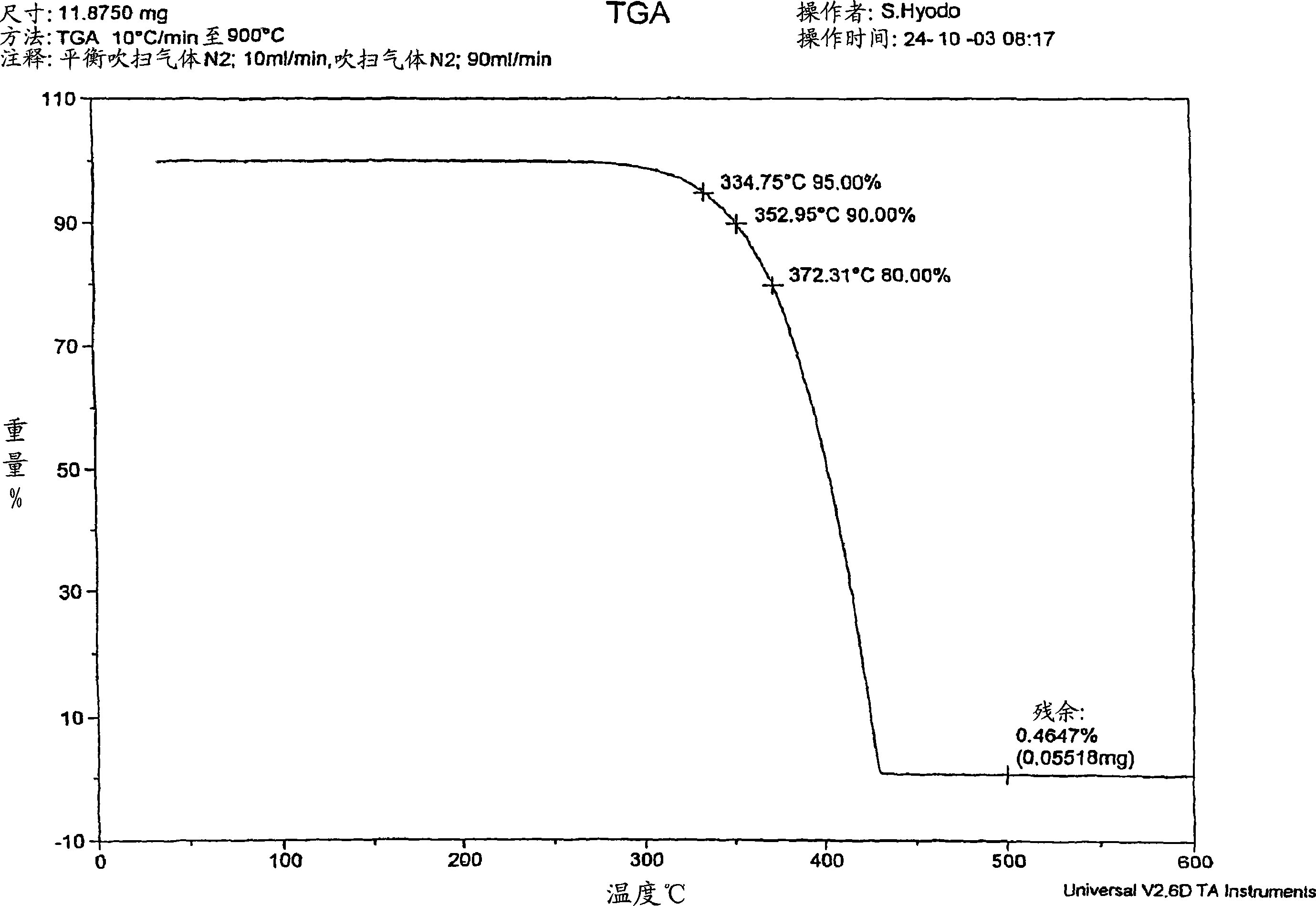
[0200] 22.55 g of 3,9-dibenzyloxy-2,4,8,10-tetraoxa-3,9-diphosphospiro[5.5]undecane ( 0.055 mol), 19.01 g (0.11 mol) of benzyl bromide, and 33.54 g (0.32 mol) of xylene were stirred at room temperature while blowing dry nitrogen. Then, heating was started with an oil bath, and the mixture was heated and stirred at reflux temperature (about 130° C.) for 4 hours. After heating, it was left to cool to room temperature, 20 mL of xylene was added, and stirred for another 30 minutes. The precipitated crystals were separated by filtration and washed twice with 20 ml of xylene. The obtained crude product and 100 ml of methanol were put into a reaction vessel equipped with a condenser and a stirrer, and refluxed for about 2 hours. After cooling to room temperature, the crystals were separated by filtration, washed with 20ml of methanol, and the obtained filtrate was heated at 1...
preparation example 3
[0203] Preparation of 2,4,8,10-tetraoxa-3,9-diphosphospiro[5.5]undecane, 3,9-dibenzyl-3,9-dioxide (FR-3)
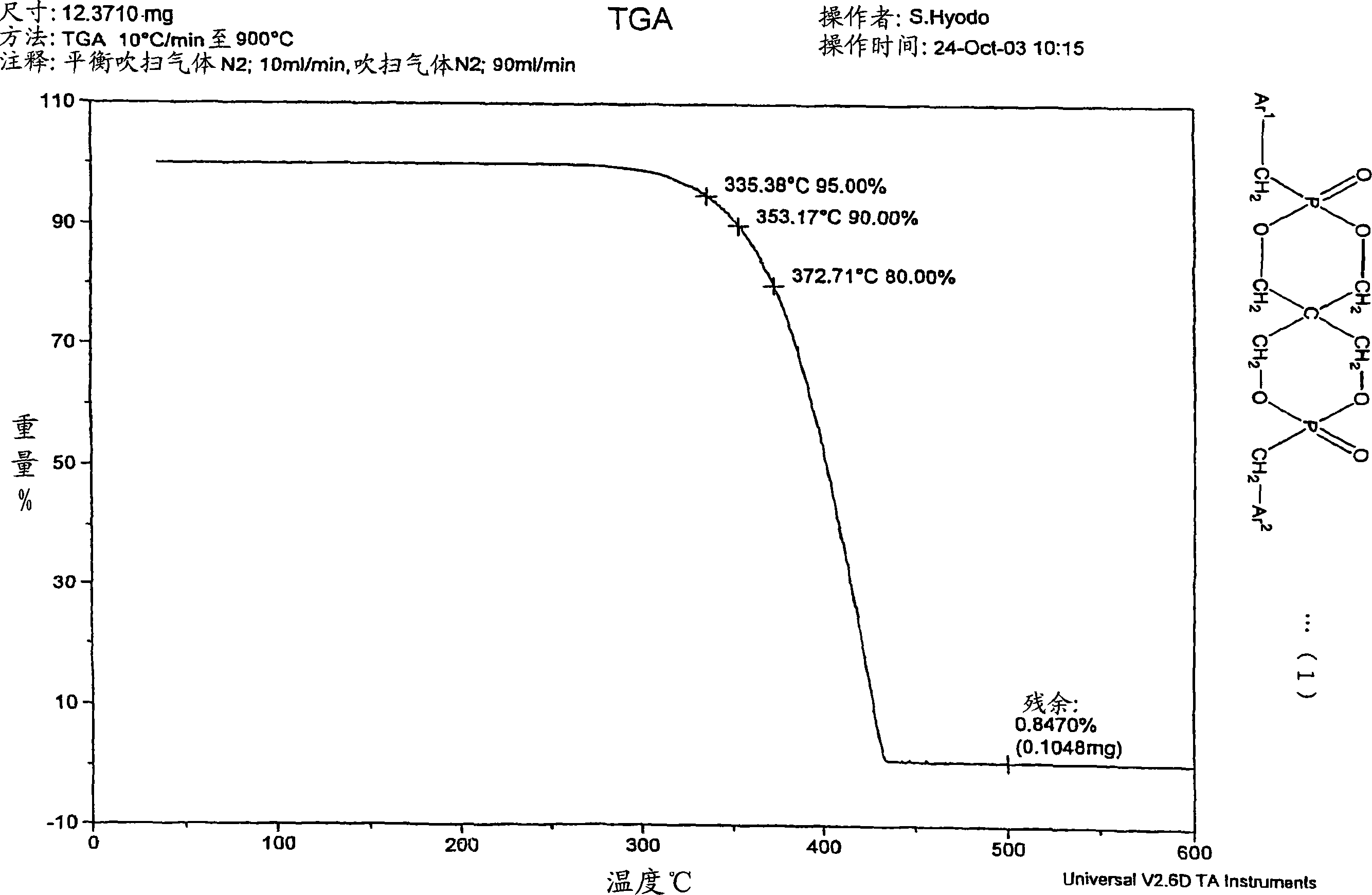
[0204] 22.55 g of 3,9-dibenzyloxy-2,4,8,10-tetraoxa-3,9-diphosphospiro[5.5]undecane ( 0.055 mol), 19.01 g (0.11 mol) of benzyl bromide, and 33.54 g (0.32 mol) of xylene were stirred at room temperature while blowing dry nitrogen. Then, heating was started with an oil bath, and the mixture was heated and stirred at reflux temperature (about 130° C.) for 4 hours. After heating, it was left to cool to room temperature, 20 mL of xylene was added, and stirred for another 30 minutes. The precipitated crystals were separated by filtration, at 100°C, 1.33×10 2 The resulting filtrate was dried under reduced pressure at Pa. The obtained white solid 1 H. 31 P-NMR confirmed that it was 2,4,8,10-tetraoxa-3,9-diphosphospiro[5.5]undecane, 3,9-dibenzyl-3,9-dioxide. Heating weight loss residue is 10.1%, acid value is 2.5mgKOH / g, HPLC purity is 84%, 31 PNMR purity was 85%.
[0205]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com