Stabilization of enzymes
一种脂肪酶、酶结构的技术,应用在酶稳定、水解酶等方向,能够解决活性位点没有暴露、不易控制、不易控制颗粒尺寸等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 (not optimized) Preparation of cross-linked or stabilized lipase spheres (structure) from water-in-oil emulsions
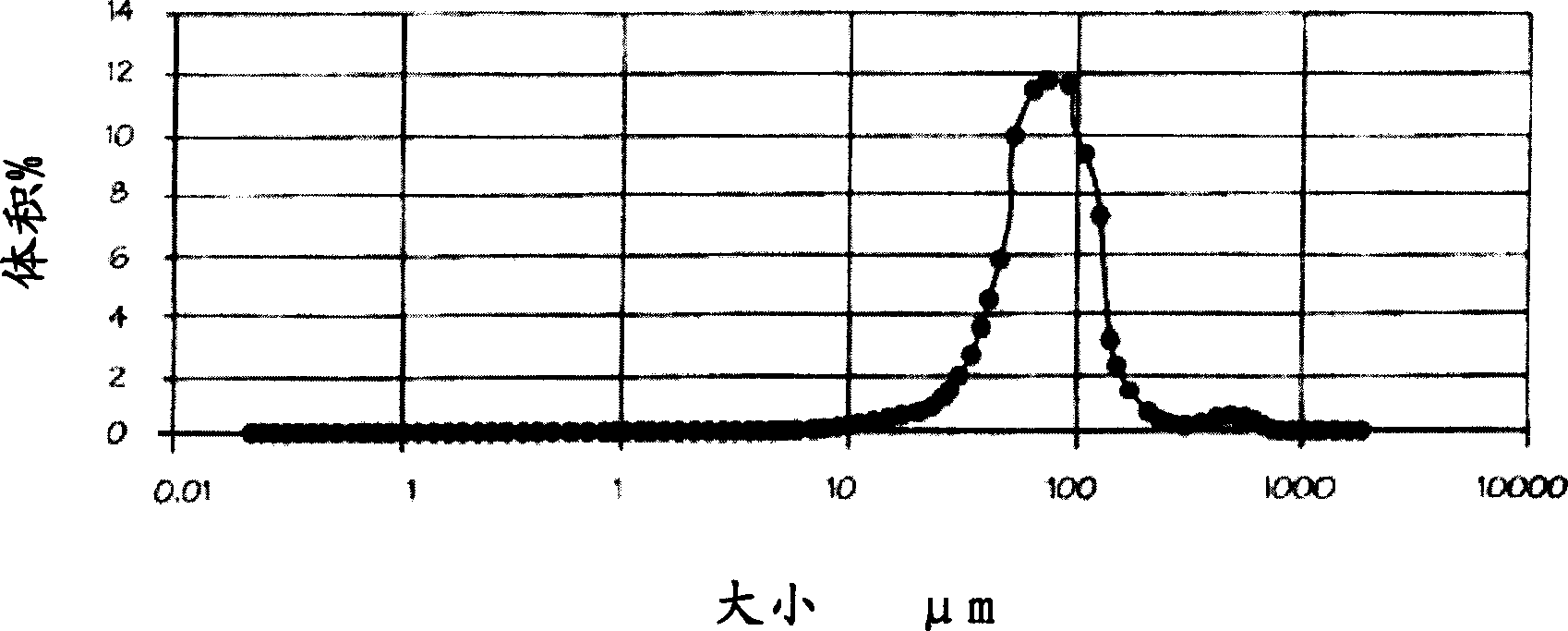
[0043] 1 g of lipase Amano AK was added to 195 g of phosphate buffered saline (PBS) solution (pH 7.8) and 5 g of mineral oil (Castrol). Homogenize the mixture using a Silverson L4R laboratory rotor-stator homogenizer at 6000 rpm for 5 minutes. 1.5 g of hexamethylene diisocyanate (Merck Schuchardt) were added to the emulsion, and the emulsion was stirred at room temperature for 2 hours. Subsequently, the cross-linked enzyme structure was recovered by filtration with 0.45 μm filter paper, and washed 5 times with PBS, each time using 50 ml of PBS (250 ml of PBS in total). figure 1 Typical stable enzyme spheres or structures obtained according to the method are shown. Using laser light scattering (Malvern Mastersizer 2000) to measure the particle size, the average diameter of the sand (Sautermean diameter) obtained is 49.4 μm (see figure 2 ).
[...
Embodiment 2
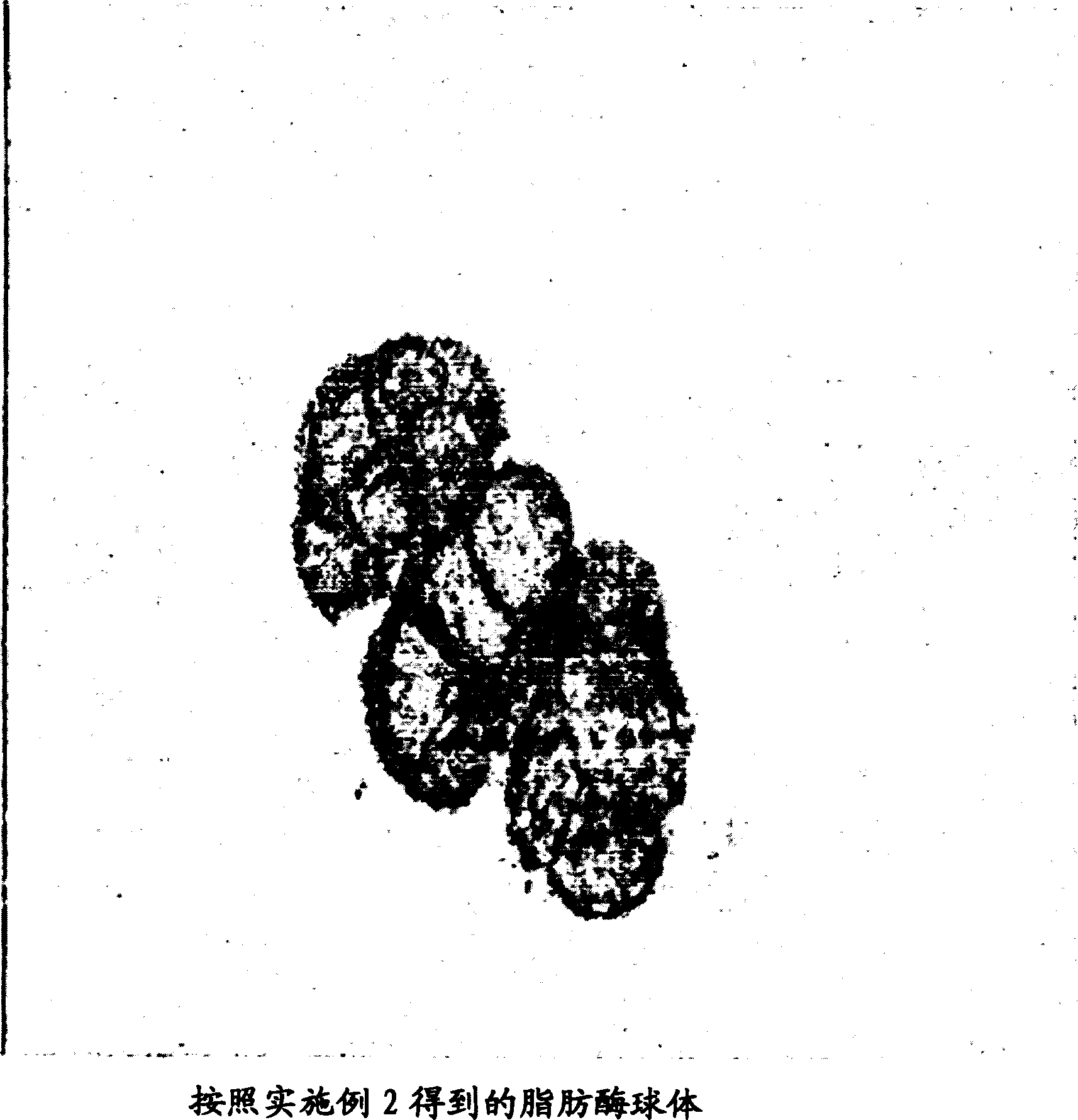
[0046] Example 2 Preparation of cross-linked or stabilized lipase spheres (structure) from water-in-oil emulsions
[0047] A lipase solution was prepared by resuspending Candida rugosa lipase (Candida rugosa lipase) (Altus Biologics, Inc) in 100 mM Tris-Cl [tris(hydroxymethyl)aminomethane] buffer (pH 8.0) to a final concentration of 100mg / ml. The enzyme was dialyzed in an Amicon ultrafiltration unit equipped with a 10K polyethersulfone membrane (Microsep (Pty) Ltd, PO Box 391647, Bramley 2018, South Africa) using 3 volumes of 100 mM Tris-Cl buffer (pH 8.0) sample.
[0048] Lipase spheres were prepared using the following volumes of the following reagents: 200 μl Candida rugosa lipase solution (prepared above); 50 μl nonoxynol-4; 50 μl tributyrin; 5 ml mineral oil. The mixture was stirred at 1500 rpm for 1 minute to emulsify. To the above solution was added 40 µl of gluteraldehyde (25% in water) and further stirred for 10 minutes. The emulsion was left at 4°C for 12 hours. ...
Embodiment 3
[0052] Example 3 Dextran aldehyde (dextran aldehyde) as a crosslinking agent
[0053] Repeat Example 2, except that the cross-linking agent used is activated dextran (dextran aldehyde) with an average molecular weight of 20 kDa from leuconostoc species, the oil phase is vegetable oil, lipase solution and Tris Example 2 was repeated except that the buffer ratio was 1:1 and no surfactant was used. Such as Hong, T., Guo, W., Yuan, H., Li, J., Liu, Y., Ma, L., Bai, Y., & Li, T. (2004) "Periodate oxidation of nanoscaled magneticdextran composites", Journal of Magnetism and Magnetic Materials, 269, 95-100, dextran aldehyde is prepared by reacting dextran with excess sodium metaperiodate. An activity of 7.5% (for p-nitrophenyl palmitate) was obtained relative to the activity of the initial free enzyme in aqueous solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com