Refining method for hexafluoromethylene aromatic compound
An aromatic compound, hexafluoromethylene technology, applied in 2, can solve problems such as large amount of wastewater, complex process, and increased environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
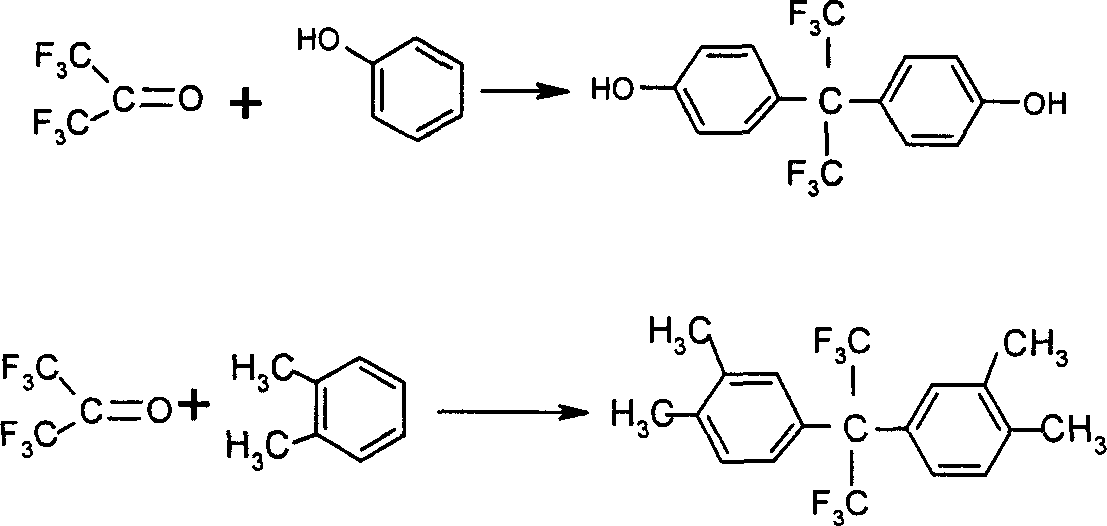
[0074] In a 1-liter autoclave, add 270 grams of xylene and 330 grams of hydrofluoric anhydride, and introduce 210 grams of hexafluoroacetone under stirring. The reaction was continued for 5 hours under the reaction conditions of 95°C / 0.9Mpa. After the reaction, put the reaction product into 3 kg of water, then fully wash the precipitated solid with water, and then dry it to obtain light yellowish brown 2,2-bis(3,4-dimethylphenyl)hexafluoropropane Crude product with a purity of 97.5%.
[0075] Add 400 grams of 20.0 grams of tan-colored 2,2-bis(3,4-dimethylphenyl)hexafluoropropane at a temperature of 70° C. and 30 grams of acetone in water (the weight ratio of acetone to water is 1:11.7; the weight ratio of 2,2-bis(3,4-dimethylphenyl)hexafluoropropane to the mixed solvent is 1:19.5). Slowly and uniformly lower the temperature to 10°C under the action of stirring to obtain a mixture of crystallization and composite solvent, filter, wash the crystallization with 5 times of deion...
Embodiment 2
[0077] In the same manner as in Example 1, the aromatic compound 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane was obtained; 20 grams of 2,2-bis(3 -Nitro-4-hydroxyphenyl)hexafluoropropane crude product, 30 grams of acetonitrile, 250 grams of toluene to form a mixture of 85 ° C into the three-necked flask [wherein 2,2-bis (3-nitro-4-hydroxyphenyl ) the ratio of hexafluoropropane to the mixed solvent weight is 1: 14.4, acetonitrile: toluene (weight)=1: 8.3], under the effect of stirring, slowly and uniformly cool down to 10 ℃ for 10 hours to obtain the mixture of crystallization and composite solvent , filter, and wash the yellowish crystals with 5 times of deionized water, filter again, transfer the crystals to an oven, and keep the temperature at 135°C for 10 hours to obtain a yellowish coarse powder 2,2-bis(3-nitro-4 -Hydroxyphenyl) hexafluoropropane 18.0 g, purity 99.6%, yield 92.4%.
Embodiment 3
[0079] In the same way as in Example 1, the aromatic compound 2,2-bis(4-hydroxyphenyl)hexafluoropropane was obtained; 20 grams of 2,2-bis(4-hydroxyphenyl) with a purity of 97.0% A mixture of crude hexafluoropropane, 20 grams of ethanol, 5 grams of methylcyclohexane, and 550 grams of water at 90°C was added to a 1000-milliliter three-necked flask [wherein 2,2-bis(4-hydroxyphenyl)hexafluoropropane and The weight ratio of the mixed solvent is 1: 29.7, ethanol: methylcyclohexane: water (weight) = 4: 1: 110], under the action of stirring, slowly and uniformly cool down to 20°C for 18 hours to obtain crystallization and compound The mixture of solvents is filtered, and the crystals are washed with 5 times of deionized water, and then filtered, and the crystals are transferred to an oven at a constant temperature of 135°C for 10 hours to obtain milky white needle crystals 2,2-bis(4-hydroxyphenyl) 18.5 grams of hexafluoropropane, with a purity of 99.8%, and a yield of 95.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com