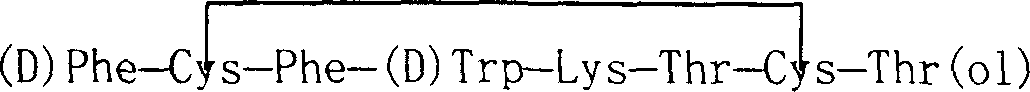Preparation method of synthesizing octriotide from solid phase polypeptide
A technology for solid-phase peptide synthesis and octreotide, which is applied in the production of peptides and bulk chemicals, and can solve problems such as environmental pollution, excessive pollution of three wastes, and low yield of peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 1. Synthetic peptide chain
[0074] (1) Preparation of Fmoc-Thr(tBu)-resin:
[0075] Take 50 grams of 2-chloro-trityl resin (100-200 mesh, 1.0mmol / g, 50mmol), soak in DMF for 30 minutes to fully swell the resin, add 44ml DIPEA, 39.7g Fmoc-Thr(tBu)- ol, reacted for 3 hours, then added 50ml of methanol to continue the reaction for 3 hours. Blow dry with nitrogen, and wash the resin three times with DMF. Obtain Fmoc-Thr(tBu)-resin;
[0076] (2) Preparation of Fmoc-Cys(Trt)-Thr-resin:
[0077] Add 800 ml of 20% hexahydropyridine in DMF and react at 25° C. for 30 minutes. Hexahydropyridine was blown and filtered with nitrogen, washed with DMF, anhydrous methanol, and DMF three times respectively, and dried with nitrogen.
[0078] Add Fmoc-Cys(Trt)-OH (MW: 585.7, 200mmol) 117.1g, TBTU (MW: 321, 200mmol) 64.2g, HOBT (MW: 153, 200mmol) 30.6g, NMM 44.4ml (MW = 101.2), 400ml of DMF, and react the mixture at 25°C for 1 hour. Blow dry with nitrogen, wash with DMF, anh...
Embodiment 2~3
[0106] Adopt the same method and processing condition as embodiment 1, wherein:
[0107] Use 4-methyltrityl resin or 4-methoxytrityl resin as the starting material respectively, connect Fmoc-Thr-ol with the same method as before, then add decapping reagent, react at 25°C for 0.5 hours, Add the mixture of Fmoc-amino acid, TBTU / HBTU and HOBT dissolved in the peptide reagent, react at 25°C for 2 hours, and then undergo reactions such as peptide cutting and oxidation to obtain 16.3g and 14.9g of white loose block products, the yields are respectively 32.0% and 29.2% (in mmol of Fmoc-Thr-resin).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com