A heavy metal ion absorbent and application thereof in removal of heavy metal ion
A technology of heavy metal ions and adsorbents, applied in the direction of adsorption of water/sewage treatment, other chemical processes, chemical instruments and methods, etc., to achieve stable effects and high removal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Preparation of magnesium-aluminum binary hydrotalcite roasted product:
[0037] The product magnesium-aluminum binary hydrotalcite obtained after peptization is divided into two parts, and roasted for 2 hours under the temperature conditions set between 400 ° C and 600 ° C, respectively, to obtain two parts of magnesium-aluminum binary hydrotalcite roasting, Grind all the synthesized products through a 160-mesh sieve, and mark the product calcined at 400°C as HT400, and the product calcined at 600°C as HT600, which becomes the heavy metal ion adsorbent.
[0038] 2. With Pb 2+ and Ni 2+ As an example, comparative experiments are used to set forth the best conditions and effects for the application of the heavy metal ion adsorbent of the present invention:
Embodiment a
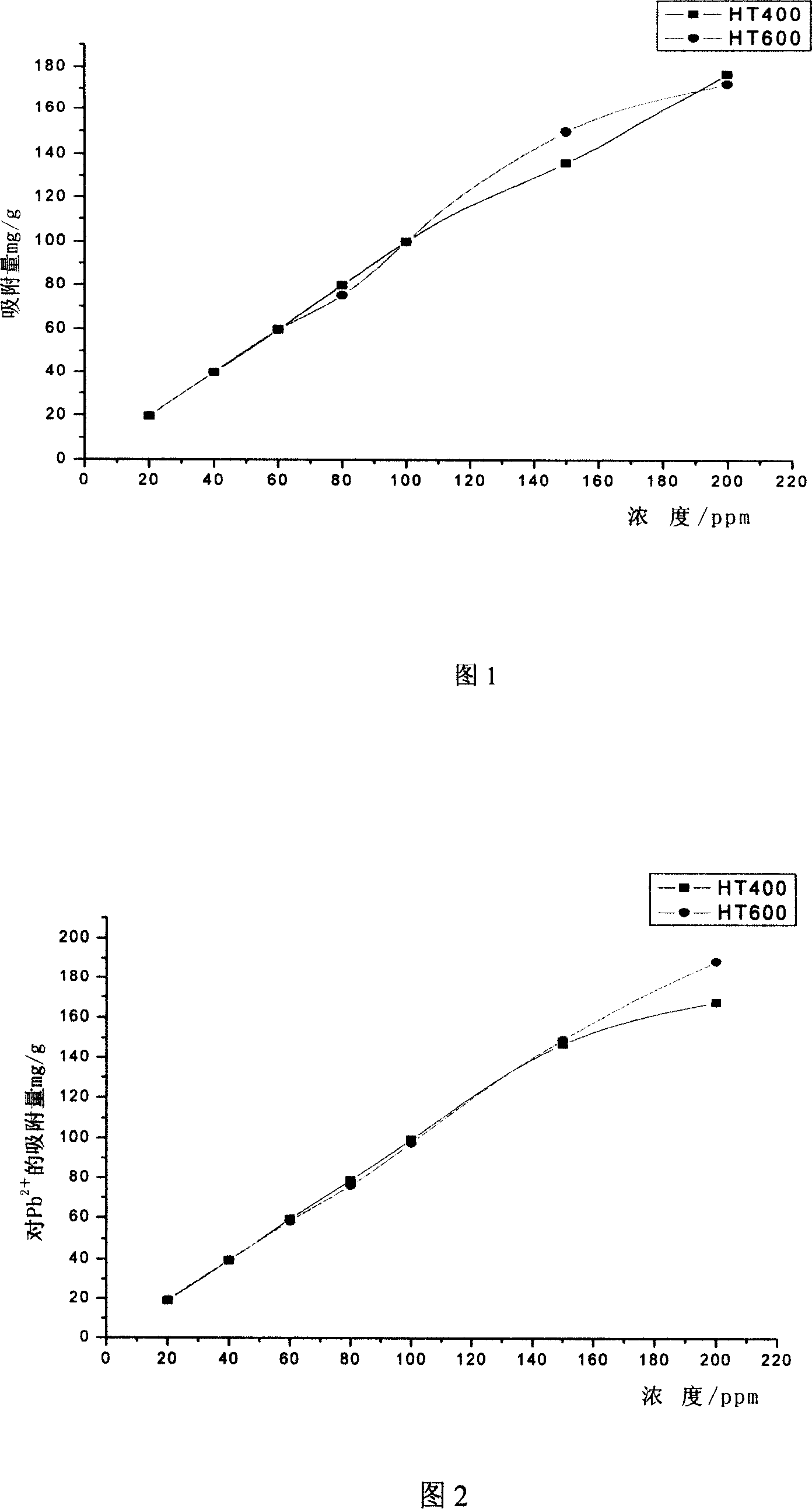
[0040] Add Ni to 28 Erlenmeyer flasks in 2 groups 2+ , Pb 2+ The initial concentration of the solution is 20, 40, 60, 80, 100, 150, 200mg / L 200ml, the dosage of both HT400 and HT600 is 200mg, and the shaking time is 2 hours. After shaking, suction filtration measures the concentration of heavy metal ions in the filtrate, and obtains the material pair Ni 2+ and Pb 2+ The adsorption effect is shown in accompanying drawing 1 and accompanying drawing 2 respectively. It can be seen from the figure that at each initial concentration, the effect of the two roasted products on Ni 2+ and Pb 2+ The adsorption effect is similar, and the removal rate is higher.
Embodiment b
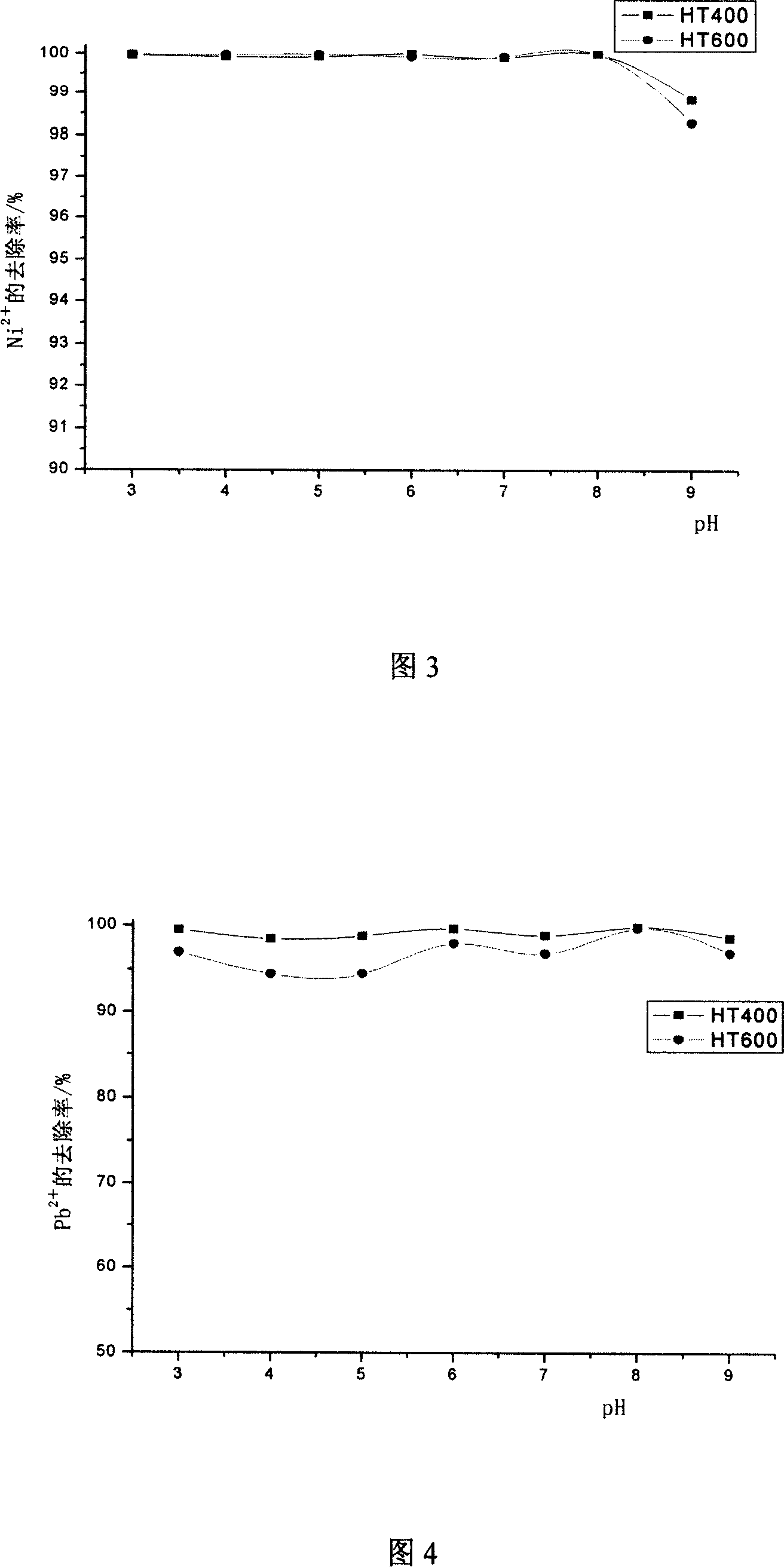
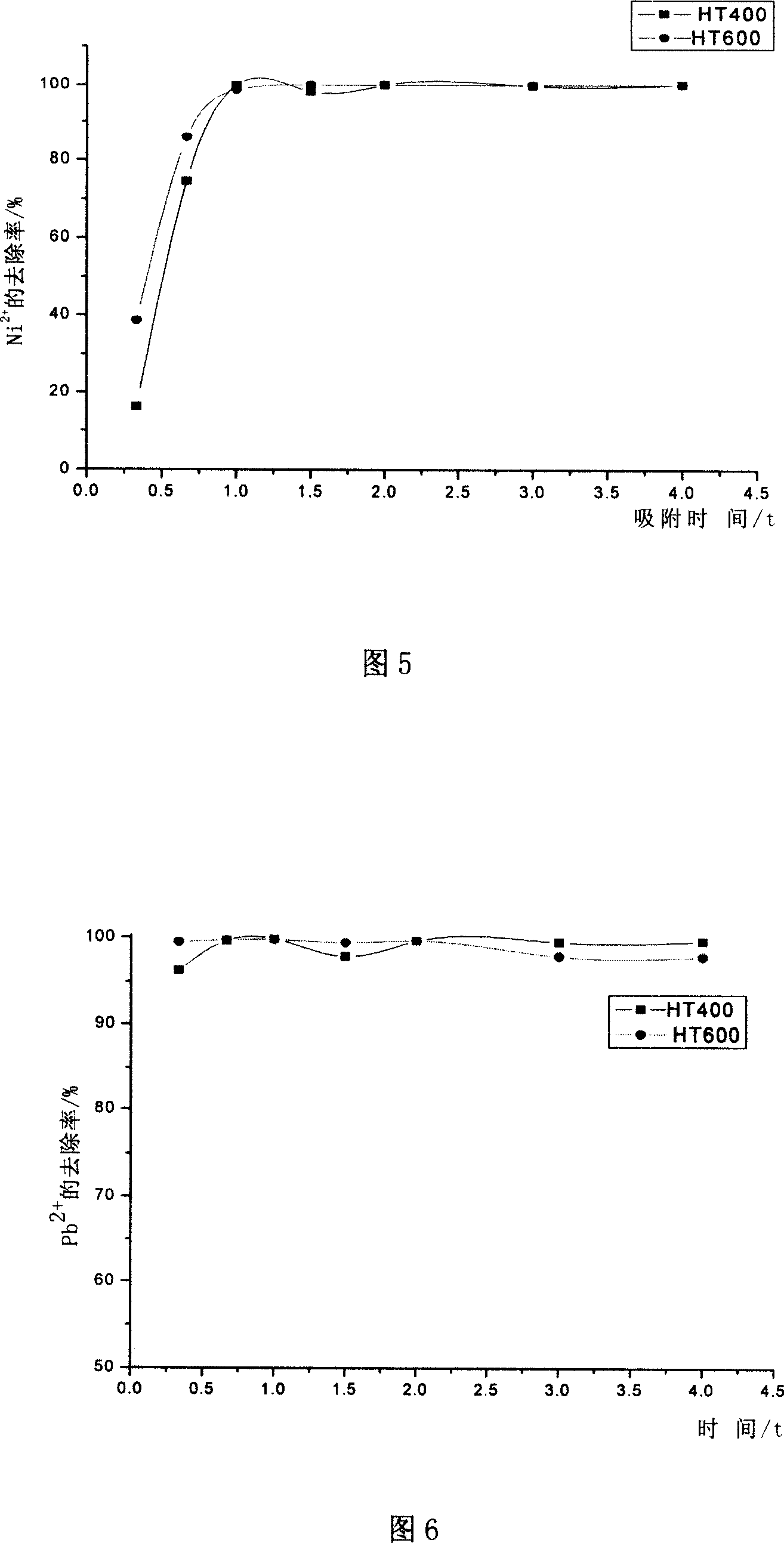
[0042] Weigh 200mg HT400 and add it into 7 groups of 200ml solutions with a certain concentration of sodium hydroxide or hydrochloric acid solution to adjust the pH value of the solution to be treated to 3, 4, 5, 6, 7, 8, 9. After shaking for 2 hours, micro Pore membrane filtration. At the same time, the parallel experiment of HT600 was done, and the material's effect on Ni 2+ and Pb 2+ The adsorption effect is shown in accompanying drawing 3 and accompanying drawing 4 respectively. It can be seen from the figure that the pH has little effect on the adsorption performance of the hydrotalcite calcined products, and the adsorption effect becomes worse when the pH is greater than 8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com