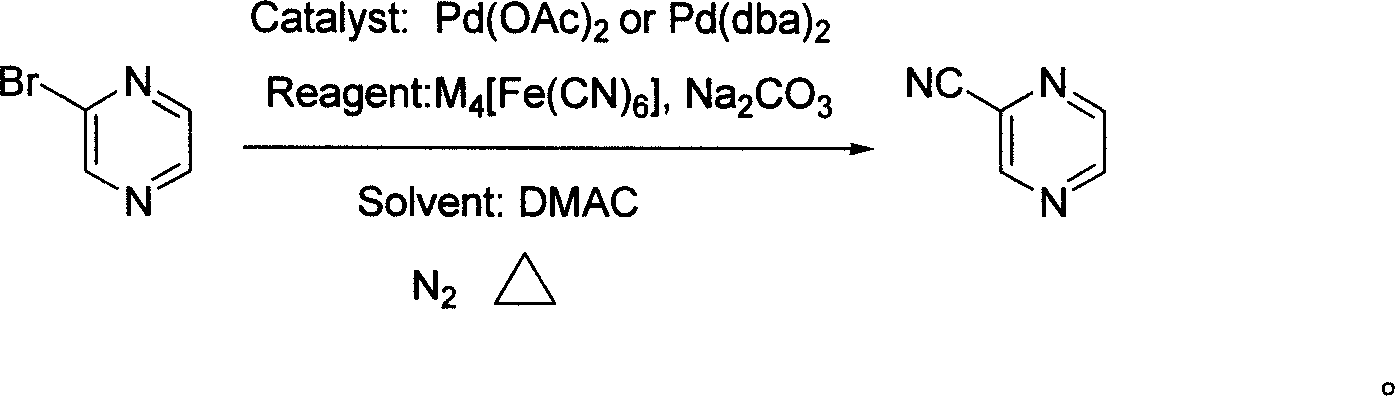
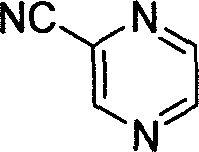
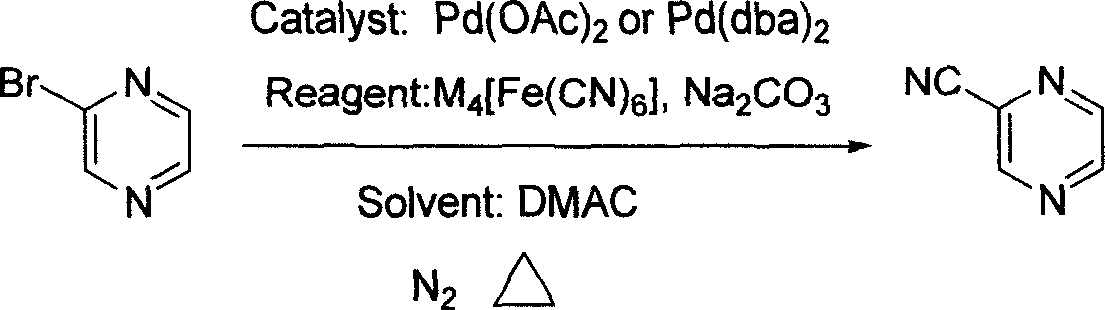Preparation process of intermediate 2-cyanpyrazine of pyrazinamide drug
A technology of pyrazinamide and cyanopyrazine, applied in the direction of organic chemistry, etc., can solve the problems of harsh conditions, high temperature and high pressure conditions, complicated post-processing, etc., and achieve the effect of simple feeding and post-processing, and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a 1000 ml three-necked flask, 500 ml of N,N-dimethylacetamide solvent, 48 g (300 mmol) of 2-bromopyrazine, 25 g (60 mmol, 0.2 equivalents) of tris Potassium ferrocyanide hydrate, 680 milligrams (3 millimoles, 0.3mol%) palladium acetate catalyst, 35 grams of sodium carbonate (330 millimoles, 1.1 equivalents), under nitrogen protection at 120 ℃ stirring reaction 2 hours, finish reaction, Then filtered, the filtrate was fractionated under reduced pressure to obtain transparent liquid 2-cyanopyrazine, boiling point: 84-87°C (18-20mmHg), yield 88%, purity 98% (GC).
Embodiment 2
[0020] In a 1000 ml three-necked flask, 500 ml of N,N-dimethylacetamide solvent, 48 g (300 mmol) of 2-bromopyrazine, 28 g (66 mmol, 0.22 equivalent) of tris Potassium ferrocyanide hydrate, 340 milligrams (15 millimoles, 5mol%) palladium acetate catalyst, 31.8 gram sodium carbonates (300 millimoles, 1.0 equivalents), under nitrogen protection, stirred reaction at 100 ℃ for 13 hours, ended the reaction, then After filtration, the filtrate was fractionated under reduced pressure to obtain transparent liquid 2-cyanopyrazine with a yield of 90% and a purity of 99%.
Embodiment 3
[0022] In a 1000 ml three-necked flask, 500 ml of N,N-dimethylacetamide solvent, 48 g (300 mmol) of 2-bromopyrazine, 20 g (45 mmol, 0.15 equivalent) of tris Sodium ferrocyanide hydrate, 1.5 millimoles (0.5mol%) two (dibenzylidene pyruvate) palladium catalysts, 31.8 grams of sodium carbonate (300 millimoles, 1.0 equivalent), under nitrogen protection, at 120 ℃ stirring reaction 4 Hours, the reaction was terminated, followed by filtration, and the filtrate was fractionated under reduced pressure to obtain a transparent liquid 2-cyanopyrazine with a yield of 80% and a purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com