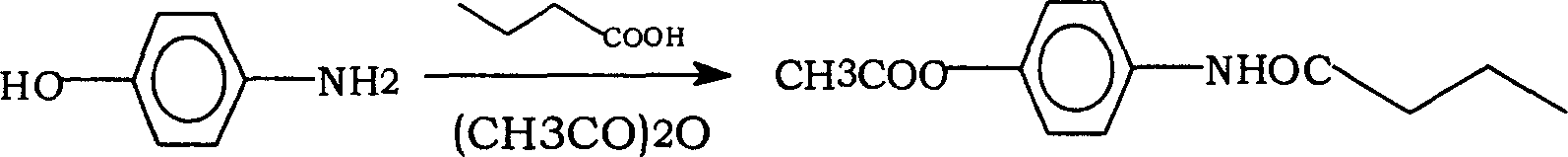Cardiovascular drug acebutolol intermediate 2- acetyl-4-n-butyramidophenol synthesis method
A technique for the synthesis of acebutolol, which is applied in the field of medicinal chemistry, can solve the problems of high cost, complex reaction operation, toxicity and pollution of reagents used, and achieve the effects of low cost, low dosage and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Put 2400g of n-butyric acid, 1000g of p-aminophenol, and 3400g of toluene into the reaction bottle, heat up and reflux for dehydration. Reflux for about 8 hours, (moisture content is about 145g) carry out atmospheric distillation and reclaim toluene. After recovering about 80% of the amount of toluene, carry out vacuum distillation to evaporate the toluene. Cool to T<35°C, add 1000g of water, stir and add 6000g of 30% aqueous sodium hydroxide solution dropwise, and then add dropwise 2200g of acetic anhydride. Stir for 2.5 hours. Cool to T<35°C and filter. Recrystallize from toluene:ethanol. Activated carbon decolorization, hot filtration. The crystalline product was dried until it had no toluene smell. Yield 65%.
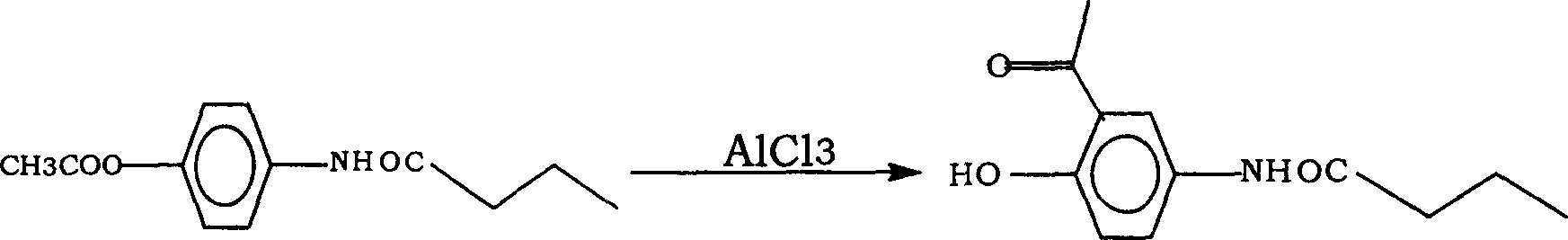
[0022] (2) Put 1400g of aluminum trichloride into the reaction bottle, stir and heat up until the internal temperature reaches 100-105°C. 800 g of 4-n-butyrylaminophenol was added in 10 times, and the temperature was raised to 110° C. for 4 hours. ...
Embodiment 2
[0024] (1) Put 2400g of n-butyric acid, 1000g of p-aminophenol, and 3400g of toluene into the reaction bottle, heat up and reflux for dehydration. Reflux for about 10 hours, (moisture content is about 146g) carry out atmospheric distillation and reclaim toluene. After recovering about 80% of the amount of toluene, carry out vacuum distillation to evaporate the toluene. Cool to T<35°C, add 1000g of water, stir and add 6000g of 30% sodium hydroxide dropwise, and then add dropwise 2200g of acetic anhydride. Stir for 2.5 hours. Cool to T<35°C and filter. Recrystallize from toluene:ethanol. Activated carbon decolorization, hot filtration. The crystalline product was dried until it had no toluene smell. Yield: 67%.
[0025] (2) Put 1400g of aluminum trichloride into the reaction flask, stir and raise the temperature until the internal temperature reaches 100-105°C. 800 g of 4-n-butyrylaminophenol was added in 10 times, and the temperature was raised to 120° C. for 4 hours. C...
Embodiment 3
[0027] (1) Put 2400g of n-butyric acid, 1000g of p-aminophenol, and 3400g of toluene into the reaction bottle, heat up and reflux for dehydration. Reflux for about 12 hours, (moisture content is about 145g) carry out atmospheric distillation and reclaim toluene. After recovering about 80% of the amount of toluene, carry out vacuum distillation to evaporate the toluene. Cool to T<35°C, add 1000g of water, stir and add 6000g of 30% sodium hydroxide dropwise, and then add dropwise 2200g of acetic anhydride. Stir for 2.5 hours. Cool to T<35°C and filter. Recrystallize from toluene:ethanol. Activated carbon decolorization, hot filtration. The crystalline product was dried until it had no toluene smell. Yield 68%.
[0028] (2) Put 1400g of aluminum trichloride into the reaction bottle, stir and raise the temperature until the internal temperature reaches 100---105°C. 800 g of 4-n-butyrylaminophenol was added in 10 times, and the temperature was raised to 140° C. for 4 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com