Preparing process of nano zinc oxide
A technology of nano-zinc oxide and sub-zinc oxide, which is applied in the fields of nanostructure manufacturing, zinc oxide/zinc hydroxide, nanotechnology, etc., can solve the problem that the final product zinc oxide particle size is difficult to control, the particle size increases, and the basic zinc carbonate crystal particle size unevenness etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
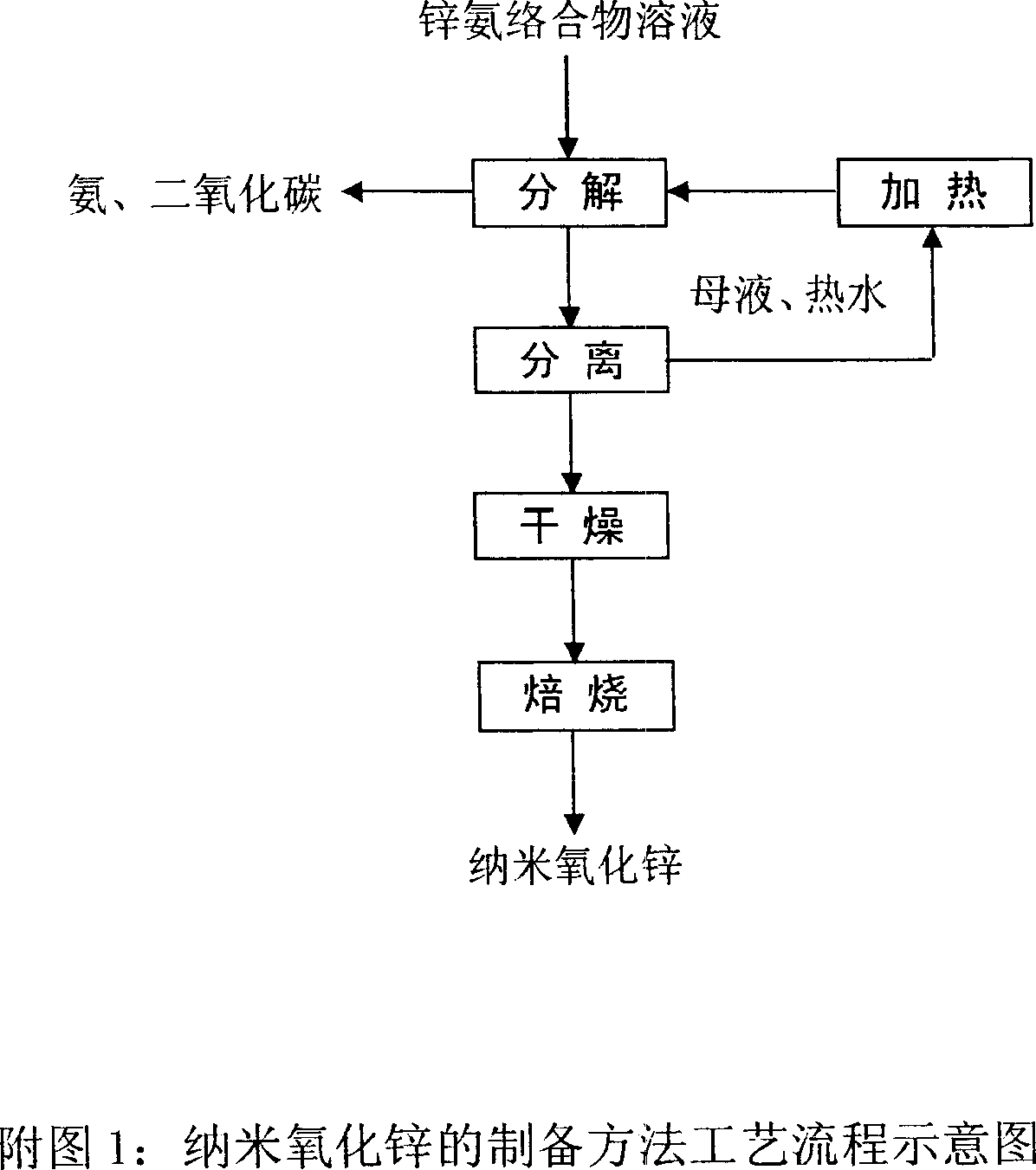
[0024] A mixed solution of zinc oxide with a content of 80% in terms of ZnO, ammonia and ammonium bicarbonate, the ratio of which is Zn:NH 3 :CO 2 =1:4:0.9 (molar ratio), stirred and reacted at room temperature for 2 hours, filtered to remove insoluble impurities, and removed metal impurities such as iron, manganese and lead through redox to obtain a purified zinc ammonia complex solution. Pump the zinc ammonia complex solution and 98°C hot water continuously into the tubular reactor at the same time, control the flow rate ratio of hot water and zinc ammonia complex solution to 8, and continuously separate the alkali from the decomposition solution Formula zinc carbonate precipitates, and the mother liquor is heated to 98°C and circulated to the reactor. The isolated basic zinc carbonate was dried and calcined at 600° C. for 2 hours to obtain a zinc oxide product with a narrow particle size distribution. As determined by transmission electron microscope and XRD line broadeni...
Embodiment 2
[0026] The zinc ammonia complex solution was obtained in the same manner as in Example 1. Pump the zinc ammonia complex solution and 90°C hot water continuously into the tubular reactor at the same time, control the flow rate ratio of hot water and zinc ammonia complex solution to 20, and continuously separate the alkali from the decomposition solution Formula zinc carbonate precipitates, and the mother liquor is heated to 90°C and circulated to the reactor. The isolated basic zinc carbonate was dried and calcined at 600° C. for 2 hours to obtain a zinc oxide product with a narrow particle size distribution. As determined by transmission electron microscope and XRD line broadening method, the average particle size of zinc oxide is 10nm, and the BET specific surface area is 62m 2 / g.
Embodiment 3
[0028] A mixed solution of zinc calcine with a content of 72% in terms of ZnO, ammonia and ammonium bicarbonate, the ratio of which is Zn:NH 3 :CO 2 =1:4.5:1 (molar ratio), stirred and reacted at room temperature for 3 hours, filtered to remove insoluble impurities, and removed metal impurities such as iron, manganese and lead through redox to obtain a purified zinc ammonia complex solution. Pump the zinc-ammonia complex solution and 95°C hot water continuously into the tubular reactor at the same time, control the flow rate ratio of the hot water and the zinc-ammonia complex solution to 5, and continuously separate the alkali from the decomposition solution Formula zinc carbonate precipitates, and the mother liquor is heated to 95°C and circulated to the reactor. The isolated basic zinc carbonate was dried and calcined at 600° C. for 2 hours to obtain a zinc oxide product with a narrow particle size distribution. As determined by transmission electron microscope and XRD lin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com