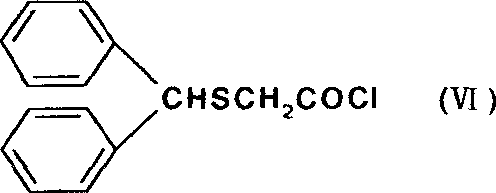
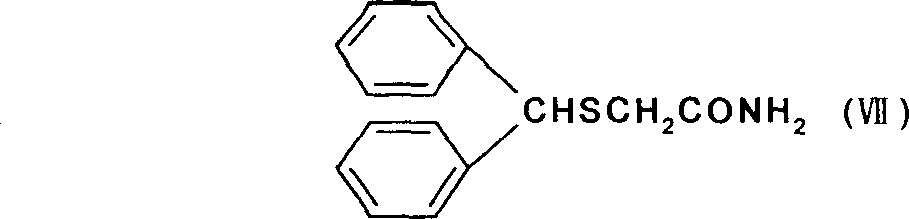
New synthesizing process for modafinil
A technology of compound and reaction temperature, applied in the field of chemical synthesis of modafinil, namely 2-acetamide, can solve the problems of long reaction route, bad smell of diphenylmethane mercaptan, complicated operation and the like, and simplify the preparation and purification methods , Product yield and quality improvement, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Preparation of Diphenylbromomethane (III)
[0045] In a 500mL three-necked flask, add 33.6g (0.2mol) of diphenylmethane, 200mL of dichloromethane, and 8g (0.2mol) of sodium hydroxide, stir vigorously, control the internal temperature at 25-30°C, and slowly add 33.6kg of bromine dropwise (0.21mol) and dichloromethane 30mL solution, the dropwise addition was completed, and the reaction was heated under reflux for 6 hours. Cool to room temperature, wash the reaction solution with 5% sodium bicarbonate solution, and then wash with water until the water layer is neutral, dry the organic layer with anhydrous sodium sulfate, recover dichloromethane under reduced pressure, and pour the oily product diphenylbromomethane into a brown bottle Store in an airtight container for later use. Yield: 98.0%.
[0046] 2. Preparation of diphenylmethylthioacetic acid (V)
[0047]In a 500mL three-necked flask, add 24.7g (0.1mol) of diphenylbromomethane and 250mL of glacial acetic acid, ...
Embodiment 2
[0055] 1. Preparation of Diphenylbromomethane (III)
[0056] In a 250 mL three-necked flask, add 33.6 g (0.2 mol) of diphenylmethane and 8 g (0.2 mol) of sodium hydroxide, and heat to an internal temperature of 95-105°C. Slowly add 33.6 kg (0.21 mol) of bromine dropwise. After the dropwise addition is complete, keep the temperature at 100-110° C. for 3 hours. Cool to room temperature, add 150 mL of dichloromethane, mix well, wash with 5% sodium bicarbonate solution, and then wash with water until the water layer is neutral, dry the organic layer with anhydrous sodium sulfate, recover dichloromethane under reduced pressure, oily product Pour diphenylbromomethane into a brown bottle and seal it for later use. Yield: 92.8%.
[0057] 2. Preparation of diphenylmethylthioacetic acid (V)
[0058] In a 500mL three-neck flask, add 24.7g (0.1mol) of diphenylbromomethane and 250mL of glacial acetic acid, stir and dissolve at room temperature, and add 8.3g (0.9mol) of thioglycolic acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com