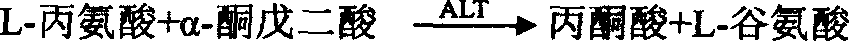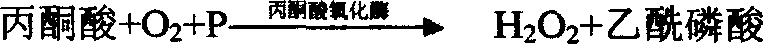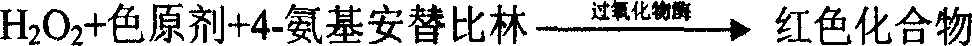Glutamate-pyruvate transaminase determination method and glutamate-pyruvate transaminase determination reagent kit
A technology of alanine aminotransferase and kit, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, measuring devices, etc. It can solve the problems of high false results, misdiagnosis and inaccuracy of clinical diseases, and achieve detection accuracy High, accurate reflection, the effect of eliminating interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the composition of reagent 1 and reagent 2 of alanine aminotransferase assay kit are as follows:
[0041] The main components of reagent 1 are as follows:
[0042] Buffer 100mmol / L
[0043] Pyruvate oxidase 2KU / L
[0044] Catalase 2KU / L
[0045] Peroxidase 3KU / L
[0046] Stabilizer 1000mmol / L
[0047] The main components of reagent 2 are as follows:
[0048] Buffer 100mmol / L
[0049] Sodium azide 30mmol / L
[0050] L-alanine 1mol / L
[0051] α-ketoglutarate 30mmol / L
[0052] 4-Aminoantipyrine 20mmol / L
[0053] TBHBA 2mmol / L
Embodiment 2
[0054] Example 2. A two-step method for eliminating interference from endogenous pyruvate The method for measuring ALT activity is as follows:
[0055] 1. On the ELISA plate, there is one reagent blank well, two standard wells, and the rest are assay wells. Add 50ul of Reagent 1 to each well.
[0056] 2. Add 20ul of distilled water to the blank wells, add 20ul of calibration serum to the standard wells, add 20ul of serum to be tested to the measurement wells, mix well and incubate at 37°C for 10 minutes.
[0057] 3. Add 50ul of Reagent 2 to each well, mix well and incubate at 37°C for 10 minutes.
[0058] 4. Add 100ul of stop solution to each well, mix well and perform colorimetry on a microplate reader. The main wavelength is 492nm, the secondary wavelength is 620nm, input the position of the reagent blank well, standard well and measurement well, input the calibration serum ALT activity concentration, adjust to zero with the reagent blank well for detection, and calculate ...
Embodiment 3
[0060] Example 3. Interference of endogenous pyruvate on samples detected by one-step method:
[0061] When serum / plasma is not separated in time after blood sample collection, the glycolysis of red and white blood cells in the sample produces endogenous pyruvate. Concentration determination interferes. The specific test is as follows:
[0062] 1. On the ELISA plate, add samples respectively: 0.125mg / ml sodium pyruvate, 0.0625mg / ml sodium pyruvate, 0.0313mg / ml sodium pyruvate, 44.5U / LALT, 0.125mg / ml sodium pyruvate+ 44.5U / LALT, 0.0625mg / ml sodium pyruvate+44.5U / LALT, 0.0313mg / ml sodium pyruvate+44.5U / LALT.
[0063] 2. Mix reagent 1 and reagent 2 at a ratio of 1:1 to make a working solution.
[0064] 3. Add 100ul working solution to each well, mix with the sample and react at 37°C for 15 minutes.
[0065] 4. Add 100ul stop solution to each well, and compare the color on a microplate reader. The main wavelength is 492nm, the secondary wavelength is 620nm, and the OD value i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com