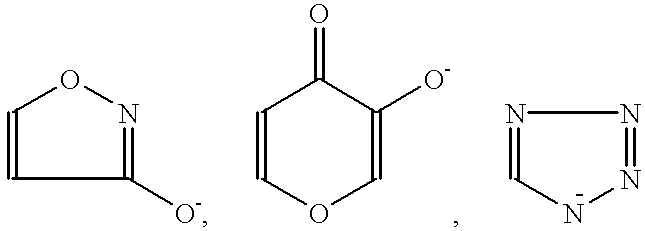
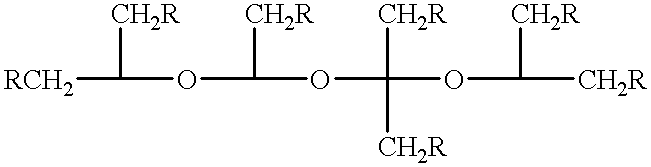
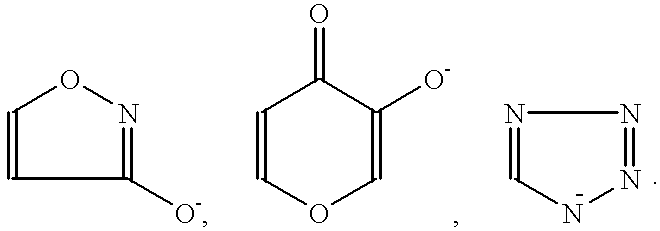
Method for treating amyloidosis
a technology for amyloidosis and amyloidosis, which is applied in the field of amyloidosis treatment, can solve the problems of no known treatment or therapy which significantly dissolves the deposits in situ, and achieves the effect of slowing down the absorption of active compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0084] The following methodologies were used:
Animals
[0085] All mice were of the CD strain (Charles Rivers, Montreal, Quebec) and weighing 25-30 g.
Animal Treatment
[0086] All animals received AgNO.sub.3 (0.5 ml, 2% solution) subcutaneously in the back, and amyloid enhancing factor (AEF) 100 .mu.g intravenously. The preparation of amyloid enhancing factor has been described previously in Axelrad, M. A. et al. ("Further Characterization of Amyloid Enhancing Factor" Lab. Invest. 47:139-146 (1982)). The animals were divided into several groups one of which was an untreated control group which was sacrificed six days later. The remaining animals were divided into those which received poly(vinylsulfonate sodium salt) (PVS) at 50 mg, 40 mg, 20 mg, or 10 mg by intraperitoneal injection every 12 hours or sucrose octasulfate ammonium salt (SOA) at 73 mg or 36.5 mg every 8 hours by IP injection. The PVS used in this and all subsequent Examples was a mixture of stereoisomers. Surviving animals we...
example 2
[0093] Swiss white mice weighing 25-30 g were given Amyloid Enhancing Factor (AEF) and AgNO.sub.3 as described previously (Kisilevsky, R. and Boudreau, L. (1983) "The kinetics of amyloid deposition: I. The effect of amyloid enhancing factor and splenectomy" Lab. Invest., 48, 53-59), to induce amyloidosis. Twenty four (24) hours later they were divided into three groups. One group served as a control and was maintained on standard laboratory mouse chow and tap water ad lib. A second group received the standard chow but its water contained 20 mg / ml of poly(vinylsulfonate sodium salt) (PVS). The third group had 50 mg / ml of PVS in its drinking water. Fluid intake in both groups was the same. All animals were sacrificed on day six (6) of the experiment, their spleens collected, prepared for sectioning, spleen sections stained with Congo red (Puchtler, H., et al. (1983) "Application of Thiazole Dyes to Amyloid under Conditions of Direct Cotton Dyeing: Correlation of Histochemical and Chem...
example 3
[0094] Since it was possible that PVS was inhibiting the hepatic synthesis of the amyloid precursor, and thus failure to deposit amyloid was due to the absence of the precursor pool, the effect of PVS on the blood level of the amyloid precursor (SAA) during the course of the experiment was determined. Animals received AEF+AgNO3 as described above and were divided into two groups. Group 1 received no further treatment. Twenty four hours later, Group 2 received 50 mg of PVS by intraperitoneal injection every 12 hours for a period of 5 days. To plot the level of SAA during this process, each animal (controls and experimentals) was bled from the tail (.noteq.25 .mu.l) each day. The SAA levels in these samples were determined by a solid phase ELISA procedure (described in Brissette, L., et al. (1989) J. Biol. Chem., 264, 19327-19332). The results are shown in FIG. 5. The open circles represent the data from the PVS-treated mice, while the triangles show the data from the non-treated anim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com