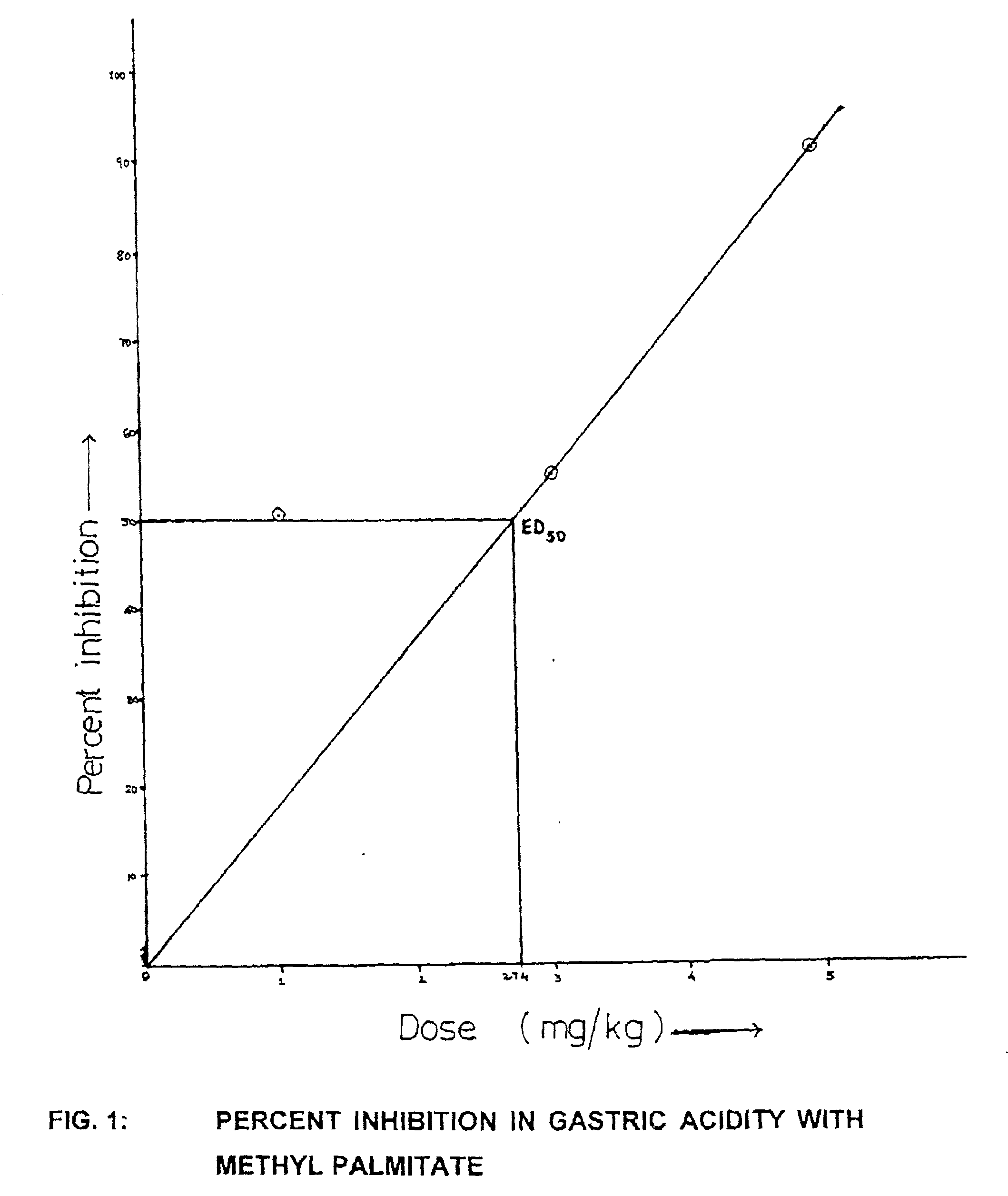
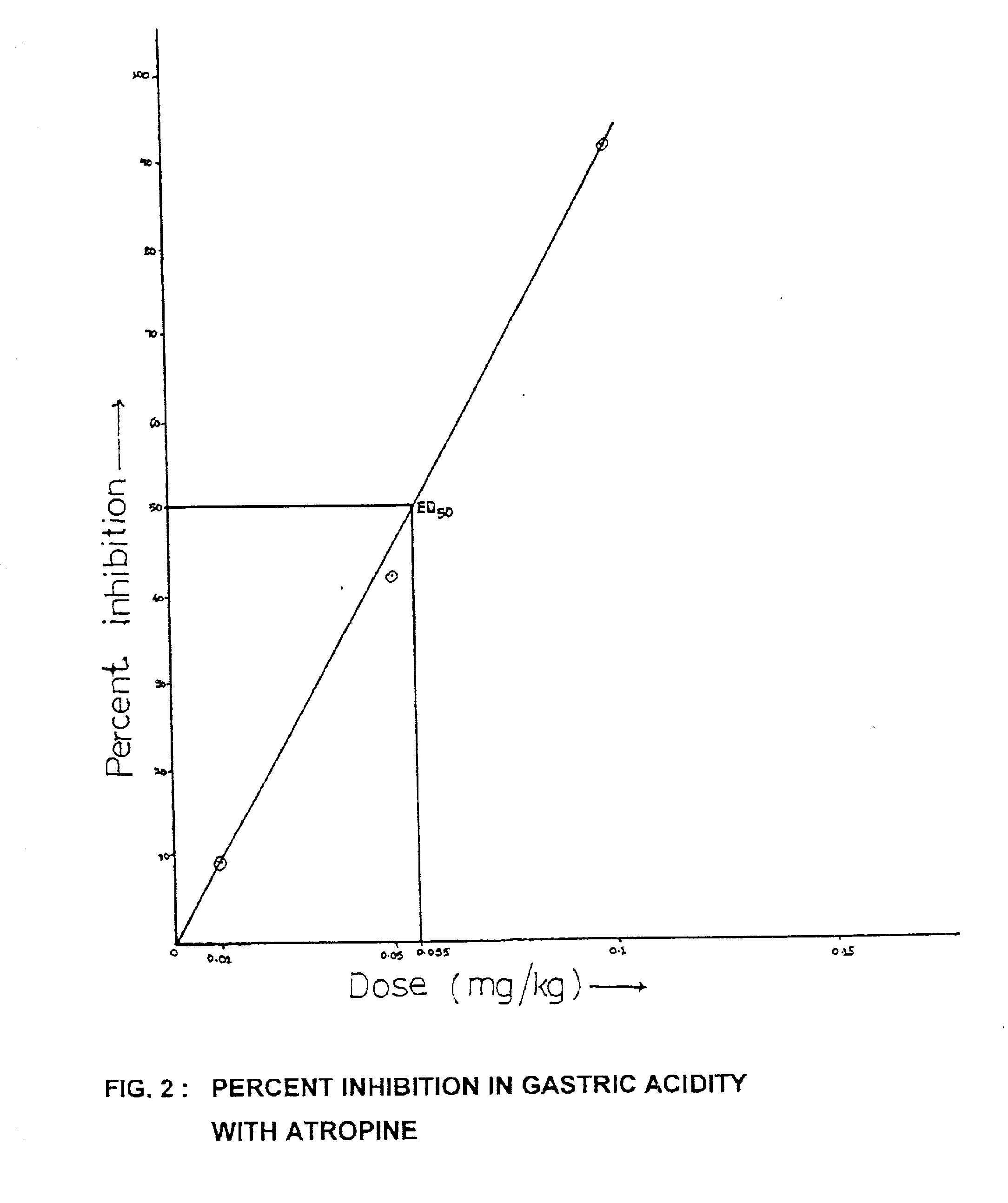
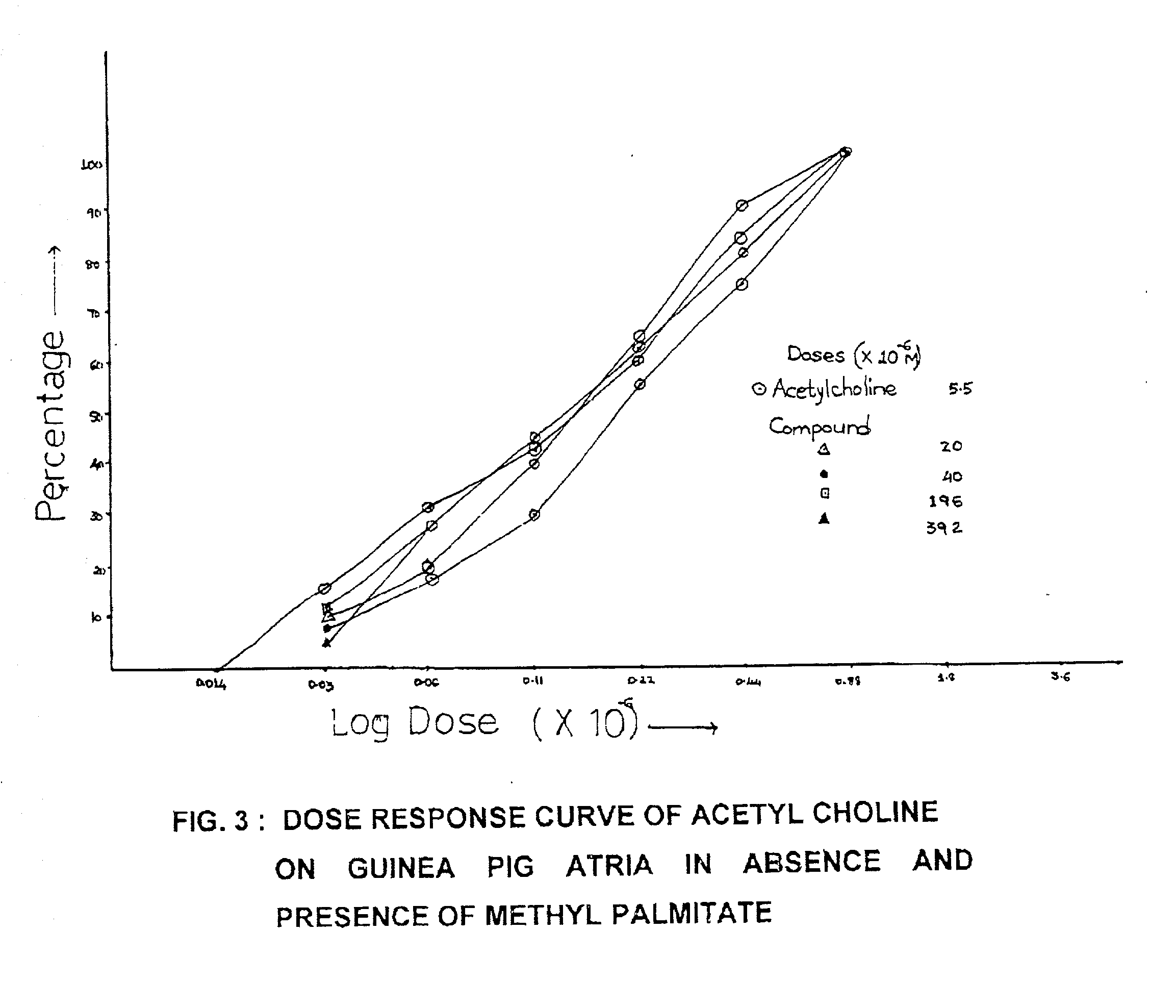
Bioactivity of methyl palmitate obtained from a mangroove plant Salvadora persica L
a technology of methyl palmitate and mangroove, which is applied in the field of bioactivity of methyl palmitate obtained from a mangroove plant salvadora persica l, can solve the problem of not having a patent available for the antimuscarinic activity of methyl palmita
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0094] Collection of the mangrove plant Salvadora persica L from the coast of Goa was along Ribandar, near the mouth of the Mandovi estuary, upstream. This species is ubiquitous to the coastal areas of Goa and was collected manually from the intertidal banks.
example 3
[0095] Processing of the collected mangroves were washed first with seawater followed by tap water. The undesired materials were sifted out while washing with tap water to get rid of the salts. The leaves, stems, and flowers of the associated mangrove plant were air dried. After drying, the plant material was cut into small pieces and immersed in the solvent 90% aqueous methanol for a week for extraction. Care was taken that these were properly soaked / dipped in the solvent so as to check putrefaction.
example 4
[0096] Extraction and preparation of crude extract was carried out by cold percolation method at room temperature and by solvent evaporation at a water bath (temperature 50.degree. C.) under reduced pressure. This helps in protection of any heat labile metabolite present in it. Re-extraction was done twice until the extract was concentrated under vacuum to obtain the crude extract.
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com