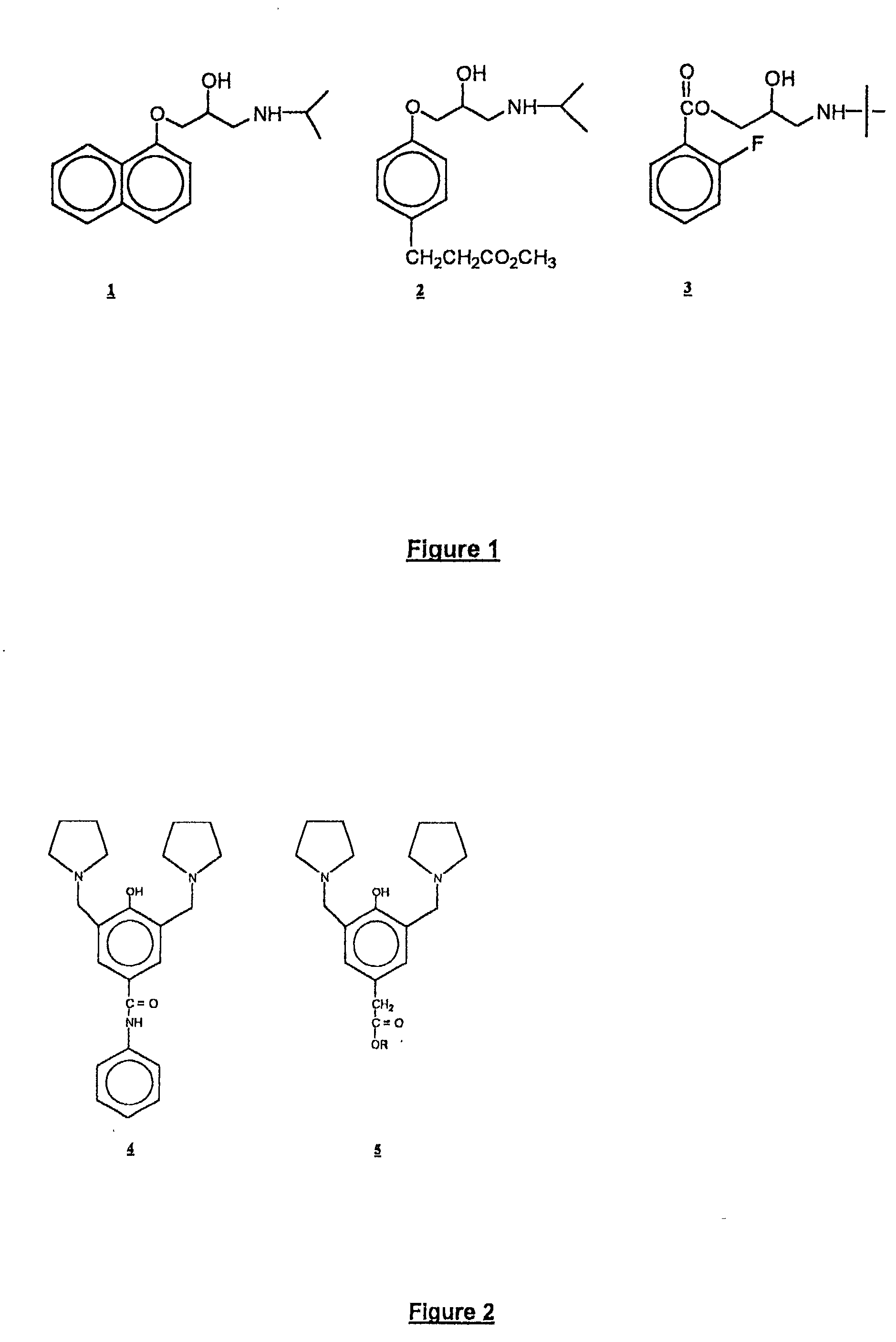
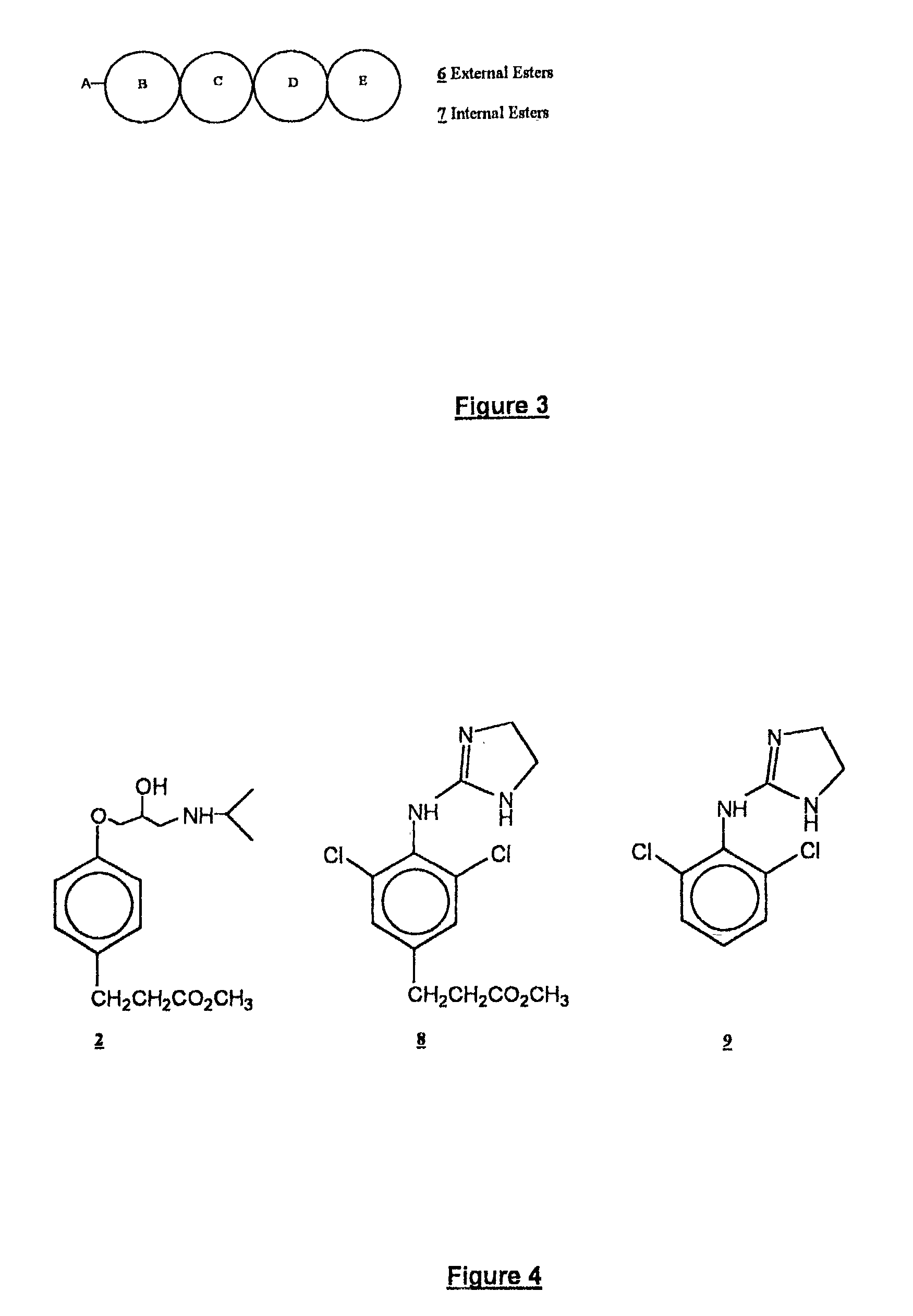
Aralkyl ester soft drugs
a technology of alkyl ester and soft drugs, applied in the direction of drug compositions, phosphorous compound active ingredients, cardiovascular disorders, etc., can solve the problems of inability to know exactly what dme and toxicity-related properties may need to be addressed, and the overall profile is less than ideal, and achieves convenient and safe deployment, prolonged steady-state levels, and the effect of convenient and safe us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0044] Structure 12 represents an analog of atropine that has an appended external ester metabophore. It has been designed for delivery as drops to the eye where it will then display its characteristic antimuscarinic properties that are useful during eye examinations for only about 30 minutes. Atropine's several hour duration is in large excess of the time typically needed to conduct a routine eye exam and chemical antidotes often need to be administered so that a patient's vision can be more quickly normalized. In addition, due to the same metabolic programming, the soft drug analog has a better systemic side-effect profile than atropine because the soft drug that is absorbed from this localized topical compartment is readily deactivated.
example 2
[0045] Structures 13 and 14 represent metabophore-containing, bulky analogs of decamethonium and pancuronium, respectively. Two external esters have been deployed in each case in order to further enhance the overall molecules' metabolic biotransformations given that these esters' close placements to the bulky aromatic rings slow their individual metabolic hydrolyses rates. The parent compounds' inherent anti-nicotinic activities, produced in a non-depolarizing fashion at neuromuscular junctions by virtue of the presence of the bulky functionalities, has a short half-life due to the appended metabophores. These compounds are ideally suited for use during surgery where there is a long-standing need for titrable, short-acting, non-depolarizing neuromuscular junction blocking agents: Approaches to Short-Acting Neuromuscular Blocking Agents: Nonsymmetrical Bistetrahydroisoquinolinium Mono- and Diesters, N. C. Dhar, R. B. Maehr, L. A. Masterson, J. M. Midgley, J. B. Stenlake and W. B. Was...
example 3
[0046] Structures 15 and 16 represent metabophore-containing analogs of prazosin and indoramin, respectively. Structure 15 contains a single sulfonate ester appendage while Structure 16 contains both an internal carboxylate metabophore and an external phosphonate ester appendage. In both cases, the inherent .alpha..sub.1-receptor antagonist properties are displayed as an ultra-short duration such that both compounds are better used in critical care settings via the intravenous route to treat hypertensive crises, shock or Raynaud's disease.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
| Toxicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com