Methods for passivating stainless steel
a stainless steel and passivating technology, applied in the direction of metal material coating process, etc., can solve the problems of citric acid passivation not reaching the desired effect and significant waste treatment cost, and achieve excellent results, reduce waste treatment costs, and reduce the redox potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparison to Nitric Acid Pickled High Chromium Steel
[0029] A single lot of type S40900 stainless steel was obtained from a stainless steel manufacturer and cut into 3".times.6" coupons for passivation tests. The coupons were identified as Series 1, 2 and 3. The manufacturer was chosen because its method of processing does not involve nitric acid and therefore has limited natural passive qualities. The alloy was chosen because it has the lowest chromium content and hence possesses the least natural corrosion resistance of all the stainless steel alloys.
[0030] A singled lot of type S40900 stainless steel, which was pickled with a nitric acid solution, was obtained from another stainless steel manufacturer and cut into 3".times.6" coupons. The coupons were identified as Series 4.
[0031] Passivation baths were prepared as follows:
1 Bath 1 50% w / w Caustic Soda 50 g / L (cleaner): 34% w / w Amphoteric sur- 50 g / L factant Bath 2 Oxalic acid dihydrate 21.8 g / L (activator): 50% w / w Caustic Soda ...
example 2
Spectroscopy Analysis
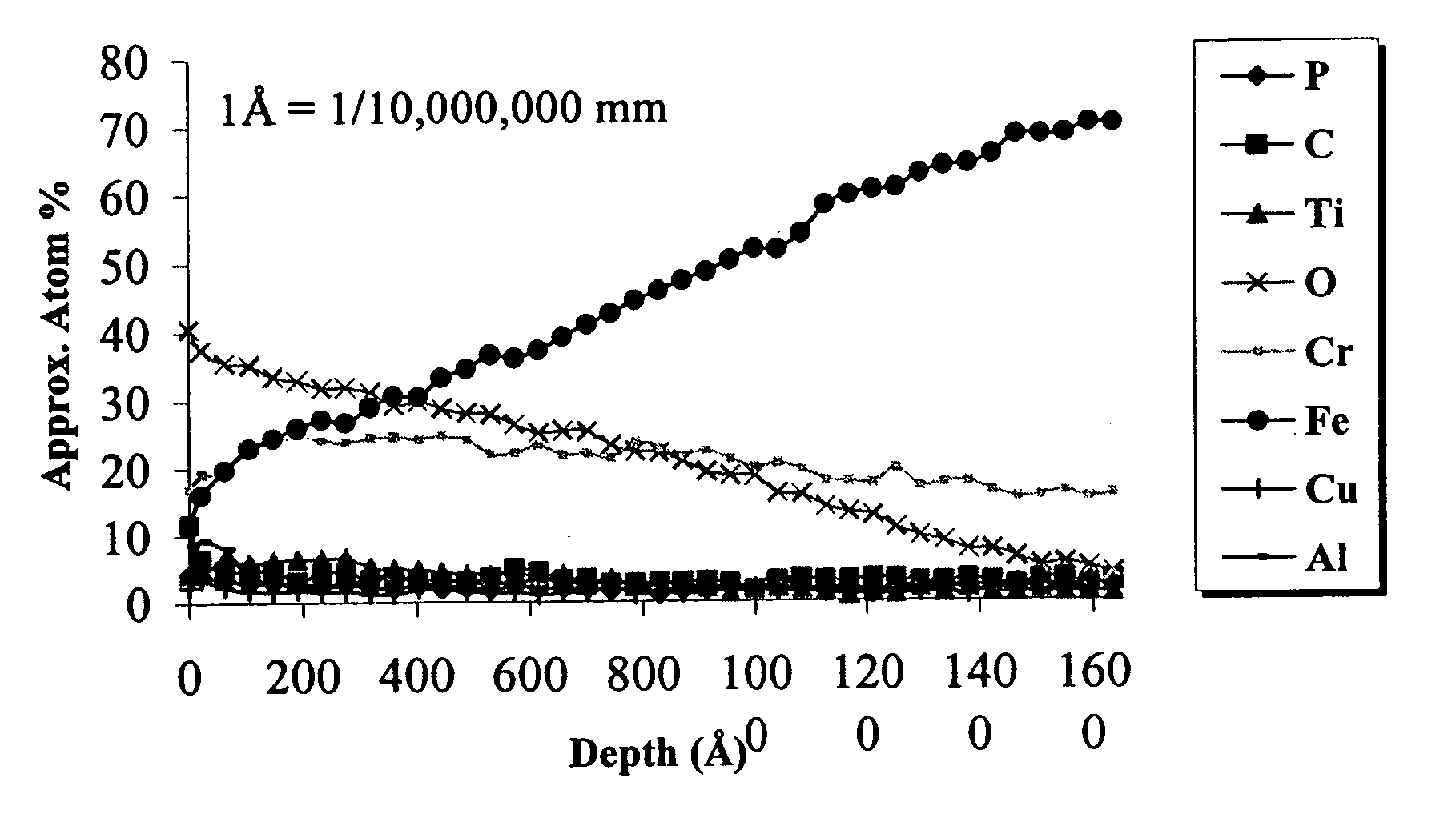
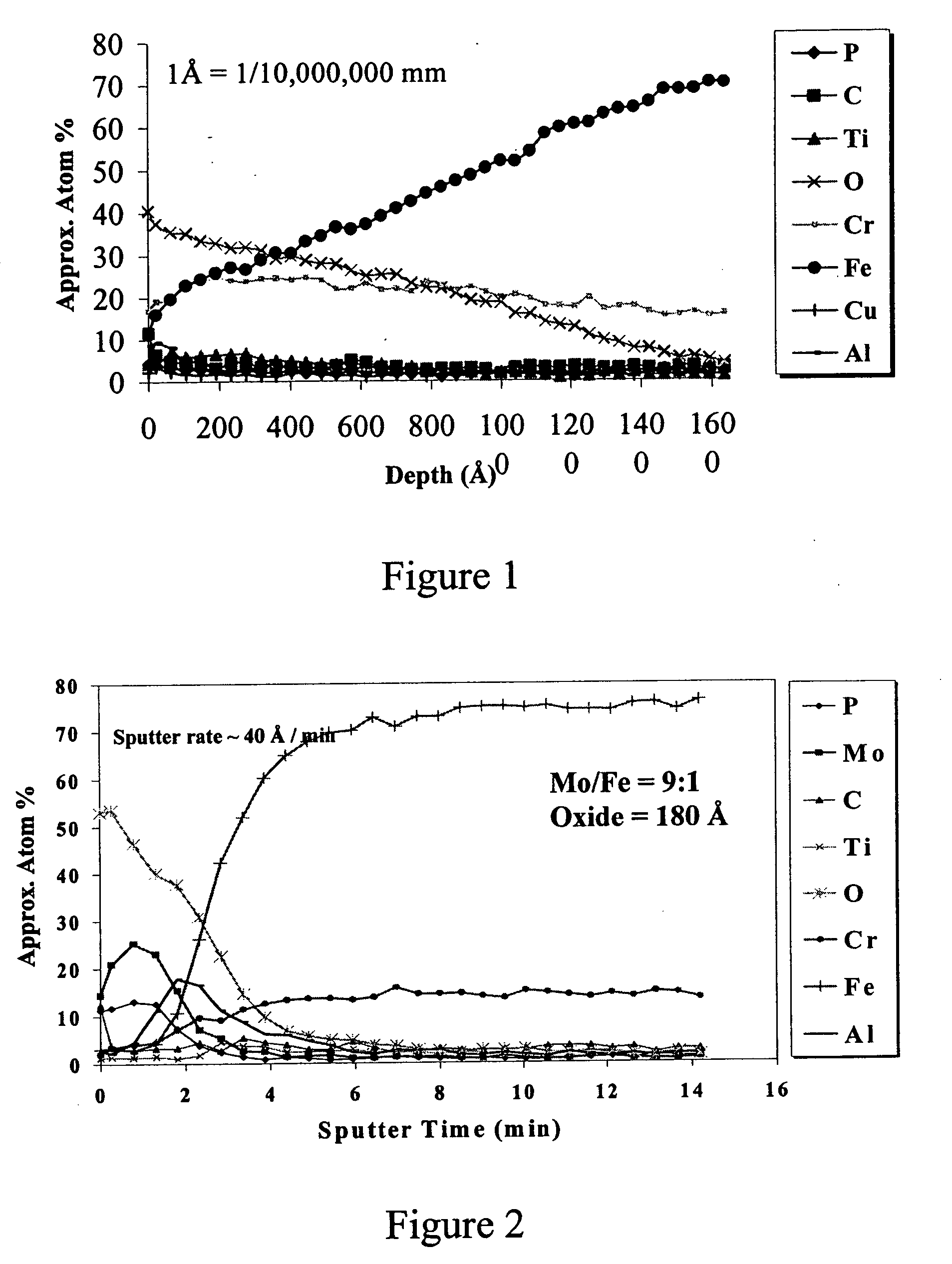
[0036] In an effort to quantify the extent of passivation afforded by the processes of this invention, samples of the coupons from Example 1 were subjected to Auger electron spectroscopy analysis to determine the chromium to iron ratios in the surface oxide layer. The Auger electron spectroscopy chart is shown in FIG. 1. As expected, the analysis yielded enriched chromium levels in the oxide layer, and chromium to iron ratios of greater than one on samples that were process treated for periods of at least one minute. In addition, the results showed that the enrichment increased as a function of treatment time, suggesting that improved corrosion protection could be realized from extended immersion times. Table 2 summarizes the typical results obtained from Auger analysis of passivation process treated samples, as well as the average passivation obtained from the ASTM A-380 treatment (described above) and the common nitric acid pickling process.
3TABLE 2 Surface Ox...
example 3
Effect of Concentration Differences
[0038] To determine the efficacy of the passivation bath (Bath 3) during periods of concentration fluctuations, a series of baths were prepared to simulate lean and rich conditions and evaluated versus the passivation treatment described previously. The results are shown in Table 3 below.
4TABLE 3 Concentration-Determined Performance Trends Perturbation Trend Reason 2.times. Phosphoric Acid Moderately negative Interference color patterns 1 / 2 Phosphoric Acid Slightly negative Corrosion performance No Sodium Fluoride Slightly negative Corrosion performance 1 / 2 Sodium Fluoride Slightly negative Corrosion performance 2.times. Sodium Fluoride Highly negative stability issue (pH) Redox potential 600 mV Moderately negative Corrosion performance Ferrous Sulfate None Equivalent corrosion performance Slightly inferior bath life
[0039] It is important to note that the importance of the iron to the passivation process is to act as a redox buffer. The hydrogen pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrochemical potential | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com