Cathode materials for secondary (rechargeable) lithium batteries
a lithium battery and cathode material technology, applied in the direction of sulfur compounds, phosphorus compounds, peroxides/peroxyhydrates/peroxyacids/superoxides/ozonides, etc., can solve the problems of reducing the output voltage of an elementary cell, limiting the power capability of the electrode, and reducing the output voltage of the elementary cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ordered Olivine LiMPO4 Compounds
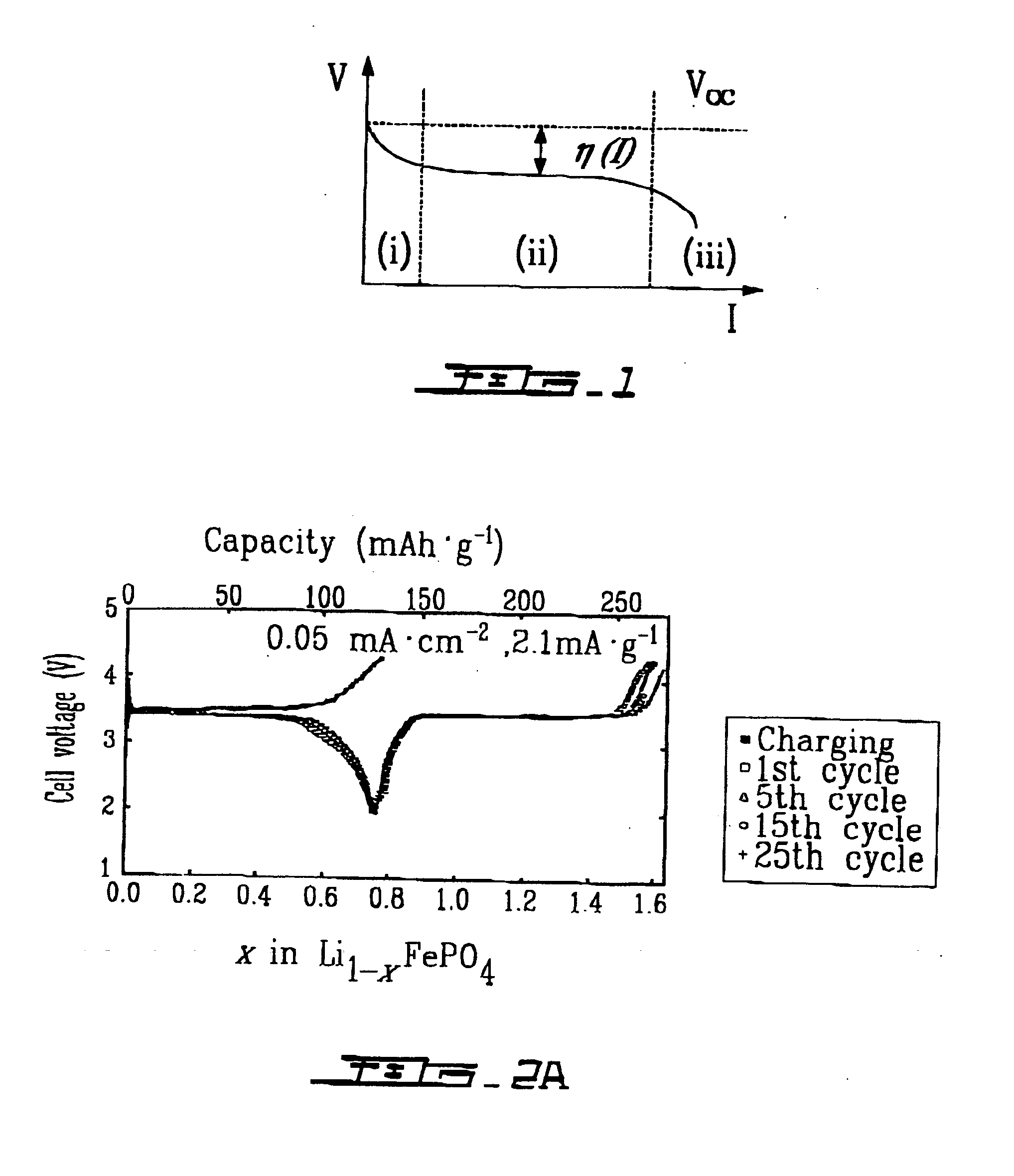
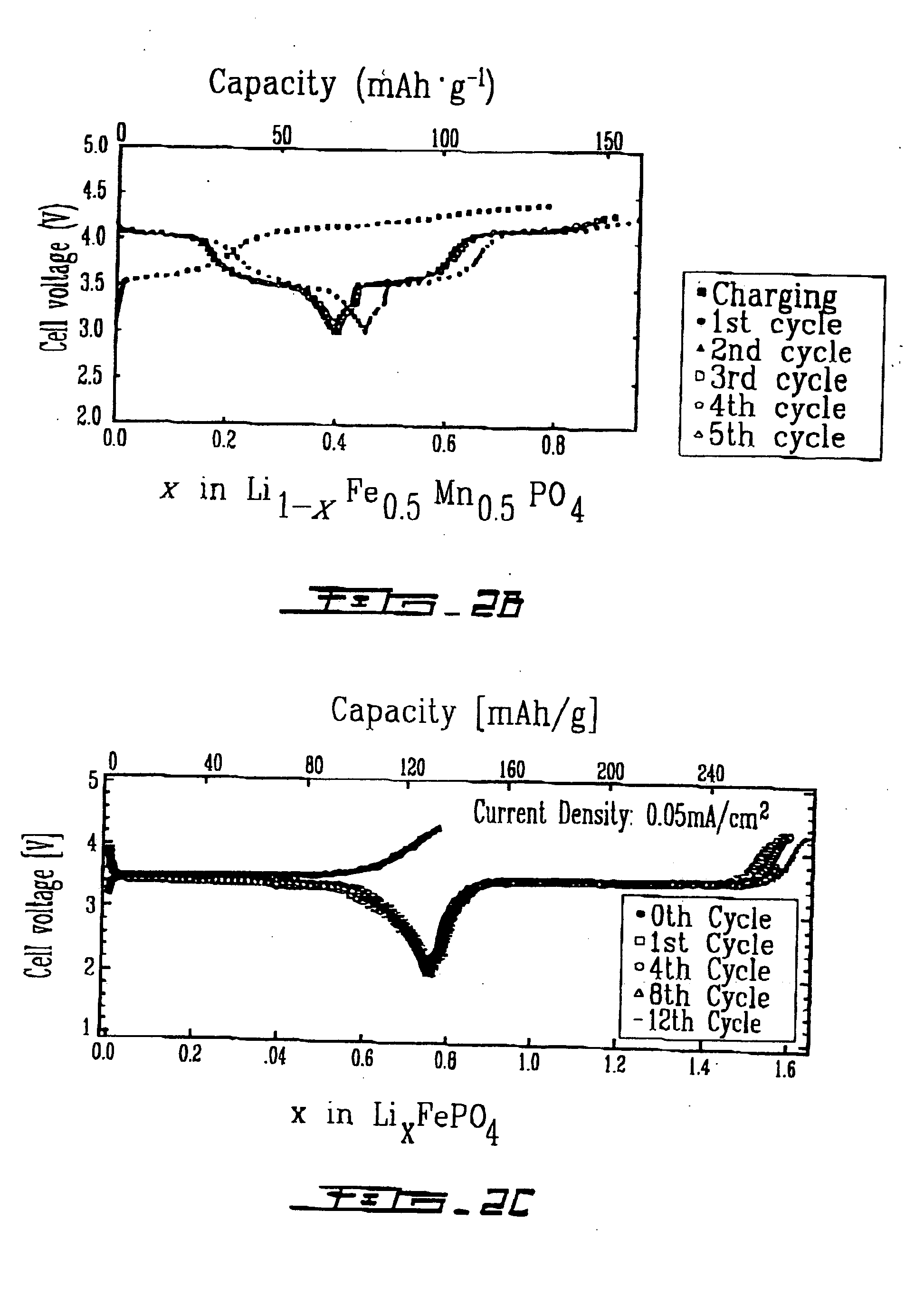
The ordered-olivine compound LiFePO4 was prepared from intimate mixtures of stoichiometric proportions of Li2CO3 or LiOH.H2O, Fe(CH3CO2)2 and NH4H2PO4.H2O; the mixtures were calcined at 300-350° C. to eliminate NH3, H2O, and CO2 and then heated in Ar at about 800° C. for 24 hours to obtain LiFe PO4. Similar solid-state reactions were used to prepare LiMnPO4, LiFe1-xMnxPO4, LiCoPO4 and LiNiPO4. FePO4 was obtained from LiFePO4 by chemical extraction of Li from LiFePO4. Charge / discharge curves for Li1-xFePO4 and discharge / charge cycles for LixFePO4 gave similar results with a voltage of almost 3.5 V vs. lithium for a capacity of 0.6 Li / formula unit at a current density of 0.05 mA·cm2− (See FIG. 2A and FIG. 2C). The electrolyte used had a window restricting voltages to V<4.3 V. Li extraction was not possible from LiNnPO4, LiCoPO4, and LiNiPO4 with the electrolyte used because these require a voltage V>4.3 V to initiate extraction. However, Li extra...
example 2
Modified Olivine LiMPO4 Compounds
The modified olivine compounds, LiMPO4, were prepared from intimate mixtures of stoichiometric proportions of Fe(C2O4).2H2O (Aldrich), LiH2PO4, Li2C2O4 (Alpha Inorganics), (NH4)2TiO(C2O4)2.H2O and polydiethoxysiloxane (Gelest) in isopropanol. In particular, 12.95 grams of iron oxalate (Fe(C2O4).2H2O), 8.31 grams of lithium dihydrogen phosphate (LiH2PO4), 1.52 grams of lithium oxalate (Li2C2O4), 2.94 grams of ammonium titanyl oxalate monohydrate ((NH4)2TiO(C2O4)2.H2O) and 2.68 grams of polydiethyoxysiloxane in 120 milliliters (ml) of isopropanol were ball-milled in a polyethylene container with sintered zirconia balls for two days. The resulting slurry was evaporated to dryness and treated in a vacuum furnace at 350° C., the 700° C. under argon (<6 ppm oxygen) to yield a compound with olivine structure of formula Li1.1Fe0.8TiP0.8Si2O4.
The compound was delithiated with a solution of bromine in acetonitrile to Li0.1Fe0.8TiP0.8Si0.2O4.
A solid-...
example 3
Rhombohedral NASICON LixM2(PO4)3 Structures
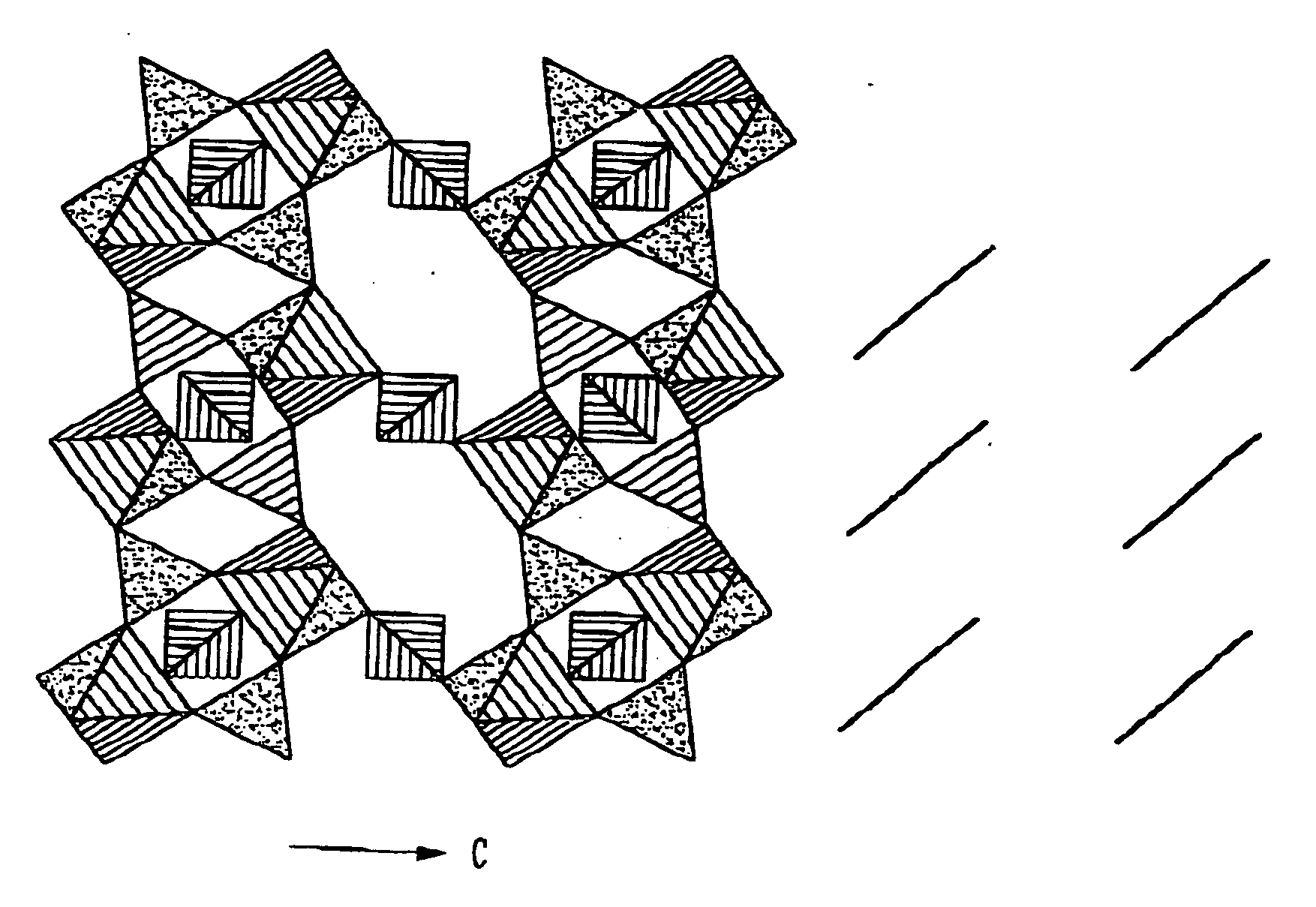
The inventors compared redox energies in isostructural sulfates with phosphates to obtain the magnitude of the change due to the different inductive effects of sulfur and phosphorus. Rhombohedral Li1+xTi2(PO4)3 has been shown to exhibit a flat open-circuit voltage Voc=2.5 V vs. lithium, which is roughly 0.8 V below the Ti4+ / Ti3+ level found for FeTi(SO4)3. The flat voltage V(x) is indicative of a two-phase process. A coexistence of rhombohedral and orthorhombic phases was found for x=0.5 (Delmas and Nadiri 1988; Wang and Hwu 1992). Li2+xFeTi(PO4)3 of the present invention remains single phase on discharge.
All three phosphates Li3M2(PO4)3, where M=Fe, Fe / V, or V, have the monoclinic Fe2(SO4)3 structure if prepared by solid-state reactionr. The inventors have found that these compounds exhibit a rhombohedral structure when prepared by ion exchange in LiNO3 at 300° C. from the sodium analog Na3Fe2(PO4)3. The discharge / charge curve of FIG. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| metallic | aaaaa | aaaaa |
| conductive | aaaaa | aaaaa |
| polymeric | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com